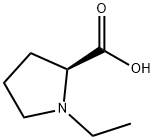
1-ethyl-L-Proline synthesis
- Product Name:1-ethyl-L-Proline
- CAS Number:98435-76-8
- Molecular formula:C7H13NO2
- Molecular Weight:143.18

64-17-5
706 suppliers
$10.00/50g
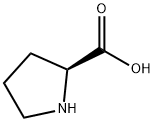
147-85-3
981 suppliers
$6.00/25g

98435-76-8
14 suppliers
$45.00/100mg
Yield:98435-76-8 100 %
Reaction Conditions:
with C26H37Cl2IrN3(2+)*2C2F6NO4S2(1-) in 2,2,2-trifluoroethanol at 90;
Steps:
General Procedure A
General procedure: Iridium complex 11 (3.2 mg, 0.005 mmol, 1 equiv.) and silver salt AgNTf2 (4.2 mg, 0.011 mmol, 2.1 equiv.)were suspended in anhydrous CH2Cl2 (1 mL), in a vial covered in aluminum foil. The reaction mixture wasstirred at room temperature for 15 minutes. The mixture was filtered off through a pad of Celite to remove theAgCl precipitated, and transferred to an oven dried pressure tube. The solvent was evaporated under vacuumand the active catalyst species used in-situ.The pressure tube impregnated with the active iridium species Ir-3- NTf2 (0.005 mmol, 2 mol%) was loadedwith dry TFE (1 mL). The corresponding amino acid (0.25 mmol, 1 equiv.) and alcohol (0.37 mmol, 1.5 equiv.)were added and the mixture was stirred at 90 °C for 20 hours. The crude was then cooled down to r.t., driedunder vacuum. Diethyl ether (3 mL) was added to the crude and the mixture was left in an ultrasonic bath for 5min. The ethereal phase containing the excess of alcohol and the catalyst was removed. The process was repeated3 more times. After drying, 1 mL of methanol to remove remaining diethyl ether was added and the crude isdried under vacuum. Quantitative isolated yields were obtained in all substrates without the need of any furtherworkup or purification (Scheme S3).
References:
Bermejo-López, Aitor;Raeder, Majken;Martínez-Castro, Elisa;Martín-Matute, Belén [Chem,2022,vol. 8,# 12,p. 3302 - 3323] Location in patent:supporting information

74-96-4
431 suppliers
$9.00/5g

147-85-3
981 suppliers
$6.00/25g

98435-76-8
14 suppliers
$45.00/100mg
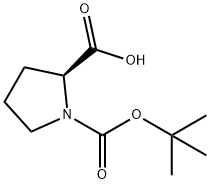
15761-39-4
603 suppliers
$5.00/10g

64-17-5
706 suppliers
$10.00/50g

98435-76-8
14 suppliers
$45.00/100mg
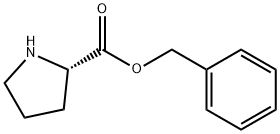
41324-66-7
8 suppliers
inquiry

98435-76-8
14 suppliers
$45.00/100mg
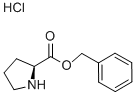
16652-71-4
321 suppliers
$6.00/5g

98435-76-8
14 suppliers
$45.00/100mg