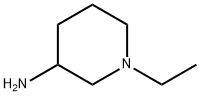
1-ETHYLPIPERIDIN-3-AMINE synthesis
- Product Name:1-ETHYLPIPERIDIN-3-AMINE
- CAS Number:6789-94-2
- Molecular formula:C7H16N2
- Molecular Weight:128.22
24059-81-2
0 suppliers
inquiry

6789-94-2
63 suppliers
$63.00/200 μmol
Yield:-
Reaction Conditions:
with hydrogenchloride at 100; for 14 h;
Steps:
80.e Example 80: N- (1-ETHYL-PIPERIDIN-3-YL)-3, 4, 5-TRIMETHOXY-N- (2-METHYL-3- PHENYL-ALLYL)-BENZAMIDE
a: Acetic anhydride, RT, then 50°C, 4 h b: Ethyl iodide, DMF, 65oC, 3 h c: Sodium borohydride, THF, 0°C to RT, 14 H d: 10% Pd/C, methanol, H2 3Kg pressure, 14 h e: HCI, 100°C, 14 H, f : 1/a-methyl cinnamaldehyde, dichloromethane 2/Sodium borohydride, methanol Scheme 21: Preparation of (1-ETHYL-PIPERIDIN-3-YL)- (2-METHYL-3-PHENYL-ALLYL)-AMINE [00337] Experimental condition analogous to Example 18 were used with (1-ethyl-piperidin-3-yl)- (2-methyl-3-phenyl-allyl)-amine 0.35 g (1.3 MMOL), 3,4, 5-trimethoxybenzoic acid 0.18 g (0.86 MMOL), thionyl chloride 0.39 g, and triethylamine 0.19 ml, in 10 ml of dichlorometane. The reaction yielded 35 mg of free amine, which was converted to the HCI salt as an off-white solid. Yield: 9%. [00338] LC-MSD, m/z for C27H36N204 [M+H] +: 453.3, [00339] 1H NMR (300 MHz, MeOD) : 5 1. 2-1.5 (m, 3 H), 1.7-2. 4 (m, 7 H), 3.0 (t, 1 H), 3.3-3. 4 (m, 2 H), 3.6 (d, 2 H), 3.6-3. 9 (m, 10 H), 4.1-4. 3 (m, 2 H), 4.3-4. 4 (m, 1 H), 6.4 (s, 1 H), 6.8 (s, 2 H), 7.2-7. 5 (m, 5 H).
References:
CHEMOCENTRYX WO2004/58705, 2004, A2 Location in patent:Page 87-88