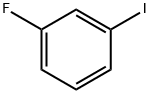
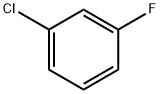
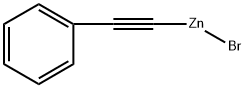1-FLUORO-3-(2-PHENYLETHYNYL)BENZENE synthesis
- Product Name:1-FLUORO-3-(2-PHENYLETHYNYL)BENZENE
- CAS Number:29778-28-7
- Molecular formula:C14H9F
- Molecular Weight:196.22
Yield:29778-28-7 96%
Reaction Conditions:
with bis-triphenylphosphine-palladium(II) chloride in tetrahydrofuran at 20; for 8 h;
Steps:
Cross-coupling reaction of 1a with 4-iodoanisole
General procedure: In a 25mL of flask containing 0.03g of Pd(PPh3)2Cl2 was added 5mL of THF. Next, 10mL (0.5M in THF, 5.0mmol) organizinc (1a) was added into the flask at room temperature. 4-Iodoanisole (0.39g, 1.67mmol) was added into the flask, then the resulting mixture was stirred at room temperature for 3.0h. Qenched with 3M HCl solution, extracted with ether (10mL×3). Combined organic layers were washed with saturated NaHCO3, Na2S2O3 solutions, and brine. Dried over MgSO4. Column chromatography with hexanes afforded 0.34g of 2a (98%) as a pale brown solid (m.p. 93-95°C).
References:
Joo, Seong-Ryu;Kim, Jong-Sung;Kim, Seung-Hoi [Tetrahedron Letters,2017,vol. 58,# 33,p. 3267 - 3270]