
1-(fluoromethyl)cyclopropanecarboxylic acid synthesis
- Product Name:1-(fluoromethyl)cyclopropanecarboxylic acid
- CAS Number:1273565-75-5
- Molecular formula:C5H7FO2
- Molecular Weight:118.11
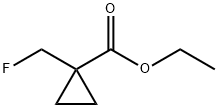
917095-87-5
17 suppliers
inquiry

1273565-75-5
28 suppliers
inquiry
Yield:-
Reaction Conditions:
with lithium hydroxide monohydrate in tetrahydrofuran;methanol;water at 20;
Steps:
Cap-3: 1-(fluoromethyl)cyclopropanecarboxylic acid
Cap-3: 1-(fluoromethyl)cyclopropanecarboxylic acid
Neat (diethylamino)sulfurtrifluoride (419 mg, 2.60 mmol) was added to a cold stirred (-78° C.) solution of ethyl 1-(hydroxymethyl)cyclopropanecarboxylate (288 mg, 2 mmol) in DCM and the mixture was warmed to rt and stirred at rt overnight.
The reaction mixture was cooled and quenched with ice cold satd. NaHCO3.
The organic layer was separated and washed with 1 N HCl, water, brine and dried (MgSO4).
Evaporation of DCM gave a light-brown oil (258 mg) which was dissolved in THF and MeOH and treated with lithium hydroxide hydrate (126 mg, 3.00 mmol) in water.
The homogeneous mixture was stirred at rt overnight and then acidified and extracted with ether to afford Cap-3 as a light brown oil. 1H NMR (400 MHz, CHLOROFORM-d) δ 4.53 (d, J=46.9 Hz, 2H), 1.51-1.45 (m, 2H), 1.13-1.07 (m, 2H).
References:
US2013/183269,2013,A1 Location in patent:Paragraph 1155; 1156
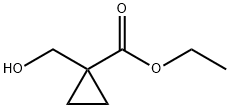
3697-68-5
87 suppliers
$28.00/100mg

1273565-75-5
28 suppliers
inquiry