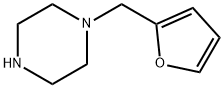
1-FURAN-2-YLMETHYL-PIPERAZINE synthesis
- Product Name:1-FURAN-2-YLMETHYL-PIPERAZINE
- CAS Number:59037-70-6
- Molecular formula:C9H14N2O
- Molecular Weight:166.22
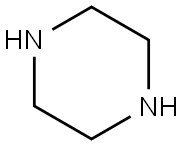
110-85-0
549 suppliers
$5.00/5G
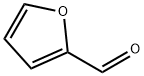
98-01-1
478 suppliers
$22.00/25g

59037-70-6
30 suppliers
$45.00/50mg
Yield:59037-70-6 58%
Reaction Conditions:
with sodium cyanoborohydride in acetic acid at 0 - 20; for 6 h;
Steps:
43.1
Step l0.5 g (5.2 mmol) of 2-furaldehyde was dissolved in acetic acid in a dried round flask provided with nitrogen gas, 2.24 g (26 mmol) of piperazine was added thereto, and 0.4 g (6.36 mmol, 1.2 eq) of sodium cyanoborohydride was slowly added thereto at 0 °C . After heating to room temperature, the reaction mixture was kept for 6 hrs, and concentrated under a reduced pressure to remove the solvent. The resulting solution was mixed with NaOH and extracted with dichloromethane. The formed organic layer was dried over anhydrous magnesium sulfate, and concentrated under a reduced pressure. The resulting residue was subjected to silica gel column chromatography (dichloromethane:methanol=4:l) to obtain l-((furan-2-yl)methyl)piperazine (yield:58%).
References:
WO2008/153325,2008,A1 Location in patent:Page/Page column 78
163839-20-1
0 suppliers
inquiry

59037-70-6
30 suppliers
$45.00/50mg

98-00-0
457 suppliers
$23.00/50g

59037-70-6
30 suppliers
$45.00/50mg

98-01-1
478 suppliers
$22.00/25g

59037-70-6
30 suppliers
$45.00/50mg