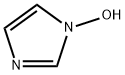
1-hydroxy-1H-imidazole 3-oxide synthesis
- Product Name:1-hydroxy-1H-imidazole 3-oxide
- CAS Number:35321-46-1
- Molecular formula:C3H4N2O2
- Molecular Weight:100.08
Yield:35321-46-1 69%
Reaction Conditions:
with hydroxylamine hydrochloride;acetic acid for 1 h;Sealed tube;Milling;
Steps:
1H-Imidazol-1-olN-oxide 1a
The glyoxal(47.0 mg, 0.810 mmol, 1.00 eq) and hydroxylamine hydrochloride (123.9 mg, 1.782 mmol,2.2 eq) were introduced in a 5 mL agate grinding jar with one agate ball (5 mmdiameter). The jar was closed and subjected to grinding for 60 min in thevibratory ball mill operated at 25 Hz. Paraformaldehyde (29.2 mg, 0.972 mmol, 1.2 eq) and AcOH (V = 100 μL, η = 0.5 μL/mg) were added and ground for next 60 min. Afterthat time, the reactionmixture was dissolved in MeOH and NH3 solution was added to pH =6-7. The solvent was evaporated and to the residue MeOH was again added. Theprecipitate of ammonium acetate was filtered and the solvent was evaporatedfrom the filtrate. The crude product was purified by column chromatography (MeOH/AcOEt,1:1, with 1% of Et3N) to afford pure 1H-imidazol-1-ol N-oxide (55.9 mg, 0.559 mmol, 69%) as colourless crystals. Mp. = 185-186 C(lit. 189 C). ADDIN EN.CITELaus1989261262617Laus,GerhardStadlwieser,JosefKl?tzer,Wilhelm1-HydroxyimidazoleDerivatives III.1,2 Synthesis of 1-Alkyloxy-, 1-Arylalkyloxy-, and1-Phenoxy-1H-imidazolesSynthesisSynthesis773-7751989107731989//&xD;02.05.20020039-7881DOI -10.1055/s-1989-27392En11H NMR (500 MHz, D2O) δ 8.30 (t, JH-H= 2.0, 1H), 7.09 (d, JH-H= 2.0, 2H) ppm.13C NMR (126 MHz, D2O) δ 124.6, 116.6ppm.HRMS (ESI/TOF) m/z:[M+H]+ calcd for C3H5N2O2101.0351; found 101.0352.IR (cm-1): 3681m, 3658, 3162m, 3135m, 3102,2979m, 2865m, 2842m, 2821m, 1453m, 1355m, 1180m, 1055s, 1028s, 1014s, 849m,776m, 722m, 591m.Anal. Calcd for C3H4F3N2O2:C, 36.00; H, 4.03; N, 27.99. Found: C, 35.78; H, 4.01; N, 27.80.
References:
Bantreil, Xavier;Lamaty, Frédéric;Lauriol, Ga?tan;Mlostoń, Grzegorz;Wróblewska, Aneta [Journal of Organometallic Chemistry,2021,vol. 949] Location in patent:supporting information
288-32-4
1040 suppliers
$5.00/25g

100-44-7
659 suppliers
$13.50/250G
121779-19-9
29 suppliers
$45.00/1mg

35321-46-1
8 suppliers
inquiry