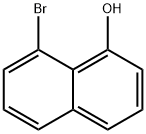
1-Hydroxy-8-bromonaphthalene synthesis
- Product Name:1-Hydroxy-8-bromonaphthalene
- CAS Number:62456-32-0
- Molecular formula:C10H7BrO
- Molecular Weight:223.07

136131-50-5
0 suppliers
inquiry

62456-32-0
53 suppliers
$127.00/100mg
Yield:62456-32-0 88%
Reaction Conditions:
with hydrogenchloride;water in ethanol at 78; for 2 h;Inert atmosphere;
Steps:
236.B Step B. 8-bromonaphthalen-l-ol.
To a solution of 5-bromo-l,4-dihydro-l,4- epoxynaphthalene (2.10 g, 1.0 eq) in EtOH (50.0 mL) was added HC1 (5.15 g, 141 mmol, 11.7 mL, 30% purity, 15.0 eq) in one portion at 25 °C under N2. The mixture was stirred at 78 °C for 2 hours. Upon completion, the reaction mixture was concentrated under vacuum. The residue was purified by reversed phase flash chromatography [Cl 8, 0.1% FA in water, 0-80% MeCN] to give 8-bromonaphthalen-l-ol (1.8 g, 88 % yield). Red oil; NMR (400 MHz, CDCb-d) d = 7.98 (s, 1H), 7.70 (dd, J= 0.8, 8.4 Hz, 1H), 7.55 (dd, J= 1.2, 7.6 Hz, 1H), 7.38-7.30 (m, 2H), 7.15 (dd, J = 8.0, 15.6 Hz, 1H), 7.00 (dd, J= 1.6, 7.2 Hz, 1H)
References:
MIRATI THERAPEUTICS, INC.;ARRAY BIOPHARMA INC. WO2021/41671, 2021, A1 Location in patent:Paragraph 0834

136131-50-5
0 suppliers
inquiry
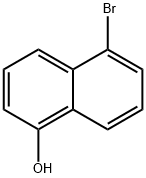
52927-23-8
43 suppliers
$107.00/100mg

62456-32-0
53 suppliers
$127.00/100mg
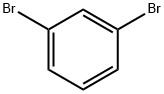
108-36-1
448 suppliers
$10.00/1g

62456-32-0
53 suppliers
$127.00/100mg
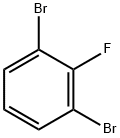
1435-54-7
79 suppliers
$10.00/100mg

62456-32-0
53 suppliers
$127.00/100mg
62456-34-2
76 suppliers
inquiry

62456-32-0
53 suppliers
$127.00/100mg