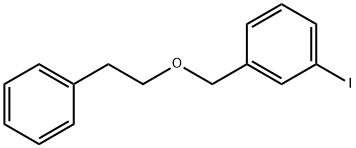
1-Iodo-3-(phenethoxymethyl)benzene synthesis
- Product Name:1-Iodo-3-(phenethoxymethyl)benzene
- CAS Number:1222138-98-8
- Molecular formula:C15H15IO
- Molecular Weight:338.18

60-12-8
553 suppliers
$6.00/25g
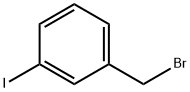
49617-83-6
169 suppliers
$6.00/250mg

1222138-98-8
3 suppliers
inquiry
Yield:1222138-98-8 99%
Reaction Conditions:
Stage #1: 2-phenylethanolwith sodium hydride in tetrahydrofuran at 25;Cooling with ice;
Stage #2: m-Iodobenzyl bromidewith tetrabutylammomium bromide in tetrahydrofuran at 25; for 20 h;
Steps:
28
Into a 100 mL round-bottom flask was placed under N2 a solution of 2- phenylethanol (110 mg, 0.90 mmol) in tetrahydrofuran (10 mL). Sodium hydride (26 mg, 1.08 mmol, 1.2 equiv.) was added with ice cooling. The mixture was stirred for 2 h at 25 0C, then 3-iodobenzyl bromide (320 mg, 1.08 mmol, 1.2 equiv.) and W-Bu4NBr (30 mg, 0.09 mmol, 0.10 equiv.) were added. The resulting solution was stirred for 20 h at 25 0C. The reaction progress was monitored by TLC (SiO2, EtO Ac/petroleum ether 1:5). The reaction was quenched by the addition of saturated aqueous NH4Cl solution. The resulting solution was extracted with EtOAc, and the organic layers were combined, dried over Na2SO4, and concentrated under vacuum. The residue was chromato graphed on silica gel with EtOAc/petroleum ether 1:20 to obtain 300 mg (99%) of l-iodo-3- (phenethoxymethyl)benzene as a yellow oil.
References:
WO2010/45212,2010,A2 Location in patent:Page/Page column 247-248