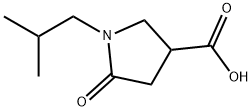
1-ISOBUTYL-5-OXO-PYRROLIDINE-3-CARBOXYLIC ACID synthesis
- Product Name:1-ISOBUTYL-5-OXO-PYRROLIDINE-3-CARBOXYLIC ACID
- CAS Number:773865-07-9
- Molecular formula:C9H15NO3
- Molecular Weight:185.22
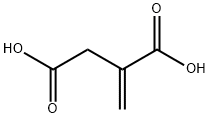
97-65-4
534 suppliers
$5.00/25g
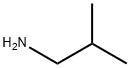
78-81-9
190 suppliers
$23.80/250ml

773865-07-9
27 suppliers
$45.00/50mg
Yield:773865-07-9 44%
Reaction Conditions:
in neat (no solvent) at 170;
Steps:
General procedure for the synthesis of 1-alkyl-5-oxopyrrolidine-3-carboxylic acids 1-4.
General procedure: A mixture of 0.01 mol of 2-methylenebutanedioic acid and 0.01 mol of an aliphatic amine was heated at 170 °C until water liberation ceased. After cooling, the reaction mixture was treated with ethanol. The precipitate was filtered off, dried, and recrystallized from ethanol. 1-Isobutyl-5-oxopyrrolidine-3-carboxylic acid (4). Yield 44%, mp 77-78 °C. IR spectrum, ν, cm-1: 1720 (C=O), 3100 (COOH), 1710 (COOH). 1H NMR spectrum, δ, ppm: 10.05 s (1H, COOH), 3.57 m (2H, C2H), 3.17 m (1H, C3H), 3.05 m [1H, (CH3)2CHCH2], 2.52 d and 2.43 d [2H, (CH3)2CHCH2, J = 4.0 Hz], 2.48 m (2, 4), 0.83 d and 0.81 d [6H, (CH3)2CHCH2, J = 4.0 Hz]. Mass spectrum, m/z (Irel, %): 199(18) [M]+, 184 (16), 156 (100), 144 (6), 127 (50), 115(4), 96 (12), 84 (9), 68 (22), 42 (24). Found, %: C 58.29; H 8.15; N 7.48. 915NO3. Calculated, %: C 58.36; H 8.16; N 7.56.
References:
Gein;Silina;Cherepanov;Shishkin;Syropyatov, B. Ya.;Voronina;Varkentin [Russian Journal of General Chemistry,2016,vol. 86,# 12,p. 2693 - 2695][Zh. Obshch. Khim.,2016,vol. 86,# 12,p. 2061 - 2063,3]