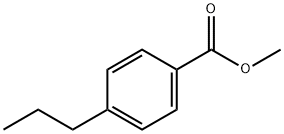
1-Methoxycarbonyl-4-propylbenzene synthesis
- Product Name:1-Methoxycarbonyl-4-propylbenzene
- CAS Number:81631-63-2
- Molecular formula:C11H14O2
- Molecular Weight:178.23
Yield:81631-63-2 97%
Reaction Conditions:
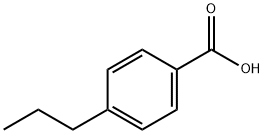
Stage #1: methanol;4-n-propylbenzoic acidwith diazomethyl-trimethyl-silane in dichloromethane at 0; for 0.583333 h;
Stage #2: with acetic acid in dichloromethane at 0;
Steps:
9A
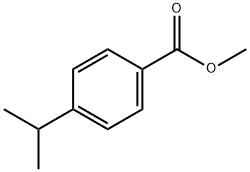
-(4-Propylphenyl)-4,5-dihydronaphtho[l,2-c]isoxazol-7-ol [00274] To a 0°C solution of 4-propylbenzoic acid (2 g, 12.18 mmol) in methanol (3.00 mL) and dichloromethane (10 mL) was added trimethylsilyldiazomethane (7.92 mL, 15.83 mmol), dropwise over a period of 5 min. Towards the end of the addition, the reaction mixture was pale yellow. The reaction was stirred at 0 °C for 30 min. and the excess diazo reagent was quenched by the slow addition of acetic acid at 0 °C (~ 1.5 mL). The colorless solution was concentrated and partitioned between ether (20 mL) and sat. aq. sodium bicarbonate (10 mL). The ether layer was washed with brine (10 mL), dried over sodium sulfate and concentrated to yield methyl 4-propylbenzoate (2.1 g, 1 1.78 mmol, 97 % yield) as a liquid. XH NMR (400 MHz, CDC13) δ ppm 7.95 (d, J=8.28 Hz, 2 H) 7.24 (d, J=8.53 Hz, 2 H) 3.90 (s, 3 H) 2.61-2.67 (m, 2 H) 1.59-1.71 (m, 1 H) 0.94 (t, J=7.40 Hz, 1 H).
References:
WO2011/59784,2011,A1 Location in patent:Page/Page column 131
2438-05-3
159 suppliers
$11.00/10g

81631-63-2
10 suppliers
inquiry

619-42-1
365 suppliers
$7.00/10g
77047-87-1
37 suppliers
$223.00/50ml
20185-55-1
48 suppliers
$35.00/1g

81631-63-2
10 suppliers
inquiry
52710-27-7
81 suppliers
$17.67/1gm:

124-41-4
681 suppliers
$12.00/25g

81631-63-2
10 suppliers
inquiry

