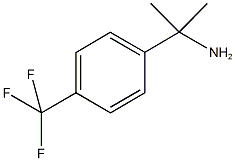
1-METHYL-1-(4-TRIFLUOROMETHYLPHENYL)ETHYLAMINE synthesis
- Product Name:1-METHYL-1-(4-TRIFLUOROMETHYLPHENYL)ETHYLAMINE
- CAS Number:306761-54-6
- Molecular formula:C10H12F3N
- Molecular Weight:203.2
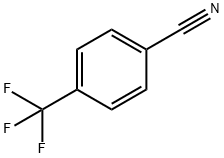
455-18-5
347 suppliers
$5.00/5g

75-16-1
270 suppliers
$12.00/10ml

306761-54-6
36 suppliers
$124.00/100mg
Yield:306761-54-6 1.81 g
Reaction Conditions:
Stage #1: 4-CF3C6H4CN;methylmagnesium bromide in diethyl ether at 20; for 0.666667 h;
Stage #2: with titanium(IV) isopropylate in diethyl ether; for 6 h;Reflux;
Stage #3: with sodium hydroxide in diethyl ether;water at 0 - 20; for 1 h;
Steps:
27-3 2-[4-(Trifluoromethyl)phenyl]propane-2-amine
Reference Example 27-3
2-[4-(Trifluoromethyl)phenyl]propane-2-amine
To a solution of 4-(trifluoromethyl)benzonitrile (3.01 g) in diethyl ether (88.0 mL), methylmagnesium bromide (about 3.0 mol/L, solution in diethyl ether, 17.6mL) was added and the mixture was stirred at room temperature for 40 minutes.
To the reaction mixture, tetraisopropyl orthotitanate (5.15 mL) was added slowly and thereafter the mixture was refluxed for 6 hours.
After cooling the mixture to 0°C, an aqueous solution of 20% sodium hydroxide was added and the mixture was stirred at room temperature for an hour.
After phase separation, the aqueous layer was extracted with diethyl ether twice.
The combined organic layers were passed through a phase separator and thereafter concentrated under reduced pressure.
The resulting residue was dissolved in 5% hydrochloric acid and washed with diethyl ether twice.
The aqueous layer was rendered basic with an aqueous solution of 20% sodium hydroxide and extracted with diethyl ether three times.
The combined organic layers were washed with saturated brine and thereafter passed through a phase separator to be concentrated under reduced pressure.
The resulting residue was purified by silica gel column chromatography (n-hexane:ethyl acetate = 95:5-10:90) to give the titled compound as a yellow oil (1.81 g).
1H NMR (300 MHz, CHLOROFORM-d) δ ppm 1.51 (s, 6 H) 7.54 - 7.69 (m, 4 H).
MS ESI/APCI Dual posi: 204[M+H]+.
References:
EP2881384,2015,A1 Location in patent:Paragraph 0411; 0412

455-18-5
347 suppliers
$5.00/5g
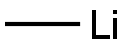
917-54-4
172 suppliers
$52.10/25ml

306761-54-6
36 suppliers
$124.00/100mg
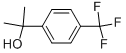
2252-62-2
46 suppliers
$42.40/1gm:

306761-54-6
36 suppliers
$124.00/100mg
402-43-7
432 suppliers
$6.00/10g

306761-54-6
36 suppliers
$124.00/100mg