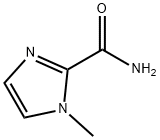
1-Methyl-1H-imidazole-2-carboxylic acid amide synthesis
- Product Name:1-Methyl-1H-imidazole-2-carboxylic acid amide
- CAS Number:20062-51-5
- Molecular formula:C5H7N3O
- Molecular Weight:125.13

30148-23-3
48 suppliers
inquiry

20062-51-5
20 suppliers
inquiry
Yield:20062-51-5 85%
Reaction Conditions:
with ammonium hydroxide in acetonitrile at 0; for 0.25 h;
Steps:
Compound 5. (Known Compound. Ref. Green Chemistry 2013, 15, 2747.)
To a stirred solution of 4 (370.1 mg, 1.63 mmol) in MeCN (3.3 mL) at 0 °C was added 25% aqueous NH3solution (3.3 mL). After 15 min, the reaction mixture was diluted with EtOAc. The organic layer was washedwith brine, dried over Na2SO4, filtered, and concentrated in vacuo. The residue was recrystallized fromn-hexane and CHCl3 to give the desired product 5 (173.3 mg, 85% yield) as white solid.
References:
Shiga, Naoki;Takayanagi, Shihori;Muramoto, Risa;Murakami, Tasuku;Qin, Rui;Suzuki, Yuta;Shinohara, Ken-ichi;Kaneda, Atsushi;Nemoto, Tetsuhiro [Bioorganic and Medicinal Chemistry Letters,2017,vol. 27,# 10,p. 2197 - 2200] Location in patent:supporting information