
1-Methyl-4-(3-Methyl-4-nitrophenyl)piperazine synthesis
- Product Name:1-Methyl-4-(3-Methyl-4-nitrophenyl)piperazine
- CAS Number:16154-61-3
- Molecular formula:C12H17N3O2
- Molecular Weight:235.28

109-01-3
653 suppliers
$5.00/5G
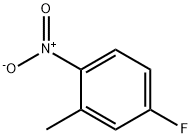
446-33-3
253 suppliers
$20.00/25g

16154-61-3
33 suppliers
$31.80/250mgs:
Yield:16154-61-3 98%
Reaction Conditions:
at 20; for 18 h;
Steps:
a (a) hl-4-3-methl-4-nitro-henlierane
(a) hl-4-3-methl-4-nitro-henlierane N-Methylpiperazine (752 pL, 6.79 mmcl) was added to 4-fluoro-2-methyl-1-nitro-benzene (527 mg, 3.39 mmcl) and the resulting mixture was stirred at room temperature for 18h. Water was added to the reaction mixture and extraction performed with ethyl acetate. Thecombined organic layers were washed with brine, dried over sodium sulfate and concentratedin vacuo. The crude product was purified by silica column chromatography (DCM/MeOH = 1/0to 9/1 v/v%) to yield the title compound (786 mg, 98%).
References:
WO2015/155042,2015,A1 Location in patent:Page/Page column 27