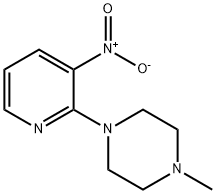
1-METHYL-4-(3-NITRO-2-PYRIDINYL)PIPERAZINE synthesis
- Product Name:1-METHYL-4-(3-NITRO-2-PYRIDINYL)PIPERAZINE
- CAS Number:5028-15-9
- Molecular formula:C10H14N4O2
- Molecular Weight:222.24
Yield:5028-15-9 94%
Reaction Conditions:
with potassium carbonate in water;Heating / reflux;
Steps:
26
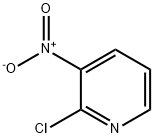
PREPARATION 26;1 -Methyl -4-(3-nitropyridin-2-yl)piperazine;.2-Chloro-3-nitropyridine (2.Og, 12.6 mmol), 1-methylpiperazine (2.1 mL, 18.9 mmol) and K2CO3 (3.5 g, 25.3 mmol) were refluxed overnight in water (50 mL). Following cooling, the mixture was extracted with EtOAc (2x75 mL). The extracts were washed with brine, dried (MgSO4) and concentrated to yield 2.63 g, 94%) of PP26 as an orange oil: MS (AP/CI) 223.0 (MH+); NMR (CDCI3) 8.29 (dd, J = 4.6, 1.7 Hz, 1 H), 8.08 (dd, J = 7.9, 1.7 Hz, 1 H), 6.70 (dd, J = 7.9, 4.6 Hz, 1 H), 3.45 (t, J = 5.2 Hz, 4H), 2.48 (t, J = 5.0 Hz, 4H), 2.31 (s, 3H); 13C NMR (CDCI3) 152.96, 151.96, 135.82, 133.17, 113.53, 54.92, 48.13, 46.36.
References:
WO2006/48727,2006,A1 Location in patent:Page/Page column 73