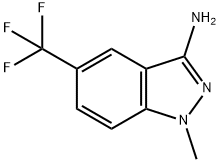
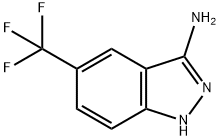
1-METHYL-5-(TRIFLUOROMETHYL)-1H-INDAZOL-3-AMINE synthesis
- Product Name:1-METHYL-5-(TRIFLUOROMETHYL)-1H-INDAZOL-3-AMINE
- CAS Number:5685-69-8
- Molecular formula:C9H8F3N3
- Molecular Weight:215.18

4088-84-0
172 suppliers
$15.00/5G
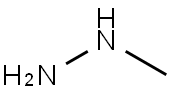
60-34-4
0 suppliers
$65.59/25g

5685-69-8
32 suppliers
$40.00/250mg
Yield:5685-69-8 67%
Reaction Conditions:
Stage #1: methylhydrazinewith sodium acetate in butan-1-ol at 25; for 0.5 h;Autoclave;
Stage #2: 2-fluoro-5-trifluoromethyl-benzonitrile in butan-1-ol at 130; for 72 h;Autoclave;
Steps:
A.4.1 Example 4: Preparation of 1-methyl-N-[1-(2-pyrimidin-2-yl-1,2,4-triazol-3-yl)ethyl]-5-(trifluoro- methyl)indazol-3-amine [I-4]:
Step 1 : In an autoclave, NaOAc (3.5g, 42.3mmol) was added to a solution of methylhydrazine (6g, 42.3mmol) in n-BuOH (5mL) at 25°C, and the resulting mixture was stirred at that tempera ture for 30min. Then, 2-fluoro-5-(trifluoromethyl)benzonitrile (4g, 21.1 mmol) was added, and the mixture was heated at 130°C for 72h. Then TLC (PE/EtOAc = 3:1, Rf = 0.2) showed that the re action was complete. The reaction mixture was quenched with H20 (50mL), the aqueous phase was extracted with EtOAc (3x50mL), and the combined organic extracts were dried over Na2S04, filtered and concentrated under reduced pressure. Trituration with MTBE (100mL), fol lowed by filtration and collection of the filter cake afforded 1-methyl-5-(trifluoromethyl)indazol-3- amine (3g, 67%) as a white oil. 1H-NMR (CDCh, 400MHz, RT): d ppm 7.85 (s, 1H), 7.55 (dd, J=8.8, 0.9Hz, 1H), 7.28 (d, J=7.9 Hz, 1H), 4.00-4.28 (m, 2H), 3.89 (s, 3H).
References:
WO2021/228594,2021,A1 Location in patent:Page/Page column 29; 33
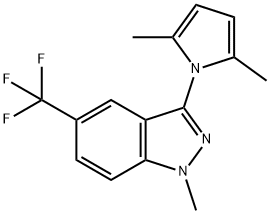
943247-23-2
0 suppliers
inquiry

5685-69-8
32 suppliers
$40.00/250mg
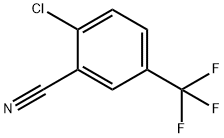
328-87-0
202 suppliers
$8.00/5g

60-34-4
0 suppliers
$65.59/25g
2250-53-5
51 suppliers
$15.00/100mg

5685-69-8
32 suppliers
$40.00/250mg