
1-(Methylsulfonyl)-3-azetidinecarboxylic acid synthesis
- Product Name:1-(Methylsulfonyl)-3-azetidinecarboxylic acid
- CAS Number:1219828-27-9
- Molecular formula:C5H9NO4S
- Molecular Weight:179.19

1219828-27-9
42 suppliers
$190.00/5G

6638-79-5
560 suppliers
$6.00/25g
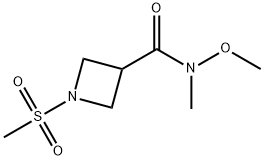
1428364-28-6
0 suppliers
inquiry
Yield:1428364-28-6 40%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine;HATU in N,N-dimethyl-formamide; for 48 h;
Steps:
A7 Example A7: l-((l-(methylsulfonyl)azetidin-3-yl)(phenyl)methyl)-lH-pyrazol-4-amine
General procedure: Example A7: l-((l-(methylsulfonyl)azetidin-3-yl)(phenyl)methyl)-lH-pyrazol-4-amine Me02S To a solution of l-methylsulfonylazetidine-3-carboxylic acid (200 mg, 1.1161 mmol, commercial) in N',N-dimethylformamide (5 mL) was added Ν,Ο-dimethylhydroxylamine hydrochloride (1.5 equiv., 1.67 mmol, 163 mg), HATU (1.5 equiv., 1.67 mmol, 636 mg) and diisopropylethylamine (3.0 equiv., 3.34 mmol, 0.59 mL). The mixture was stirred for 2 days, then diluted with 50 mL EtOAc and washed with 50 mL sat. NaHC03 (aq) and 2 x 50 mL 1 : 1 H20:brine. The organic extracts were dried (Na2S04) and concentrated in vacuo to provide N- methoxy-N-methyl-l-methylsulfonyl-azetidine-3-carboxamide (100 mg, 0.45 mmol, 40% yield) of sufficient purity to be used directly. This material was diluted with tetrahydrofuran (2 mL) and cooled to 0 °C, then phenylmagnesium bromide (3.0 mol/L) in diethyl ether (2 equiv., 0.90 mmol, 0.30 mL) was added dropwise. The mixture was stirred for 2 hours, while slowly warming to rt. The reaction was quenched by the addition of ~2 mL sat. NH4C1 (aq), then the mixture was diluted with 50 mL EtOAc and washed with 50 mL brine. The organic extracts were dried (Na2S04) and concentrated in vacuo. Purification by CombiFlash (12 g; dry load; 80:20 to 40:60 heptane:EtOAc over 20 minutes) provided (l-methylsulfonylazetidin-3-yl)-phenyl-methanone (39 mg, 0.16 mmol, 36% yield). To a solution of (l-methylsulfonylazetidin-3-yl)-phenyl-methanone (39 mg, 0.1630 mmol, 39 mg) in methanol (1 mL) and tetrahydrofuran (1 mL) was added sodium borohydride (1.5 equiv., 0.24 mmol, 9.4 mg) and the mixture was stirred for 90 minutes at rt. The reaction was quenched by the addition of ~2 mL sat. NH4C1 (aq), then the mixture was diluted with 50 mL EtOAc and washed with 50 mL brine. The organic extracts were dried (Na2S04) and concentrated in vacuo to provide (l-methylsulfonylazetidin-3-yl)-phenyl-methanol (39 mg, 0.1616 mmol, 99% yield). This alcohol was converted to an aminopyrazole via a Mitsonobu reaction followed by palladium on carbon reduction as is outlined in Example A3.
References:
WO2014/23258,2014,A1 Location in patent:Page/Page column 59; 60