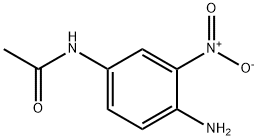
1-N-ACETYL-3-NITRO-P-PHENYLENEDIAMINE synthesis
- Product Name:1-N-ACETYL-3-NITRO-P-PHENYLENEDIAMINE
- CAS Number:6086-29-9
- Molecular formula:C8H9N3O3
- Molecular Weight:195.18
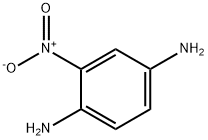
5307-14-2
331 suppliers
$5.00/10g

108-24-7
5 suppliers
$14.00/250ML

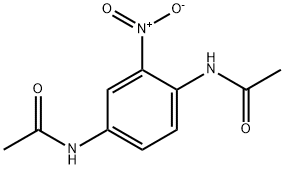
6086-29-9
24 suppliers
$57.50/500mg
Yield:6086-29-9 90%
Reaction Conditions:
in acetone;
Steps:
1.B 1 -Amino -2-nitro-4-acetamidobenzene
1 ,4-diamino-2-nitrobenzene (2-nitro- 1,4-phenylenediamine) was selectively acetylated according to the method of McFarlane et al., J. Chem. Soc. Perkin Trans., 691 (1988) incorporated herein by reference. The amine meta to the nitro group is readily acetylated using acetic anhydride in acetone (the amine ortho to theintro group is strongly deactivated). The yield of 1-Amino-2-nitro-4-acetamidobenzene (2- nitro-4-acetamido aniline) was> 90%. Characterization: ‘H NMR (CD3OD) d [ppm]: 8.3 (m, 1 H, ArH), 7.5 (M, 1 H, ArH), 6.9 (M, 1 H, ArH), 2.1 (s, 3 H, acetyl CH3) in good agreement with McFarlane. JR (nujolINaCl) n [cm’]: 3470 (s, str, HOAc), 3340-3150 (m, mlstr, acetamide ArNH + ArNH2), 1661 (s, str, acetaniide CO), 1643 (s, str, H bondedacetamide CO), 1592 (s, mlw, aryl stretch), 1547 (s, str, ArNO2) & 1512 (s, mArNO2). Anal. (Dried at 80 °C) Calcd for C8H9N30,: C, 49.23; H, 4.65; N, 21.53. Found: C, 49.36; H, 4.55; N, 21.31.
References:
WO2017/53564,2017,A1 Location in patent:Page/Page column 82
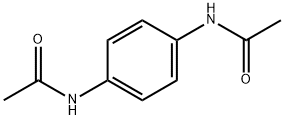
5345-53-9
35 suppliers
$62.00/1g

6086-29-9
24 suppliers
$57.50/500mg
140-50-1
104 suppliers
$20.00/10mg

6086-29-9
24 suppliers
$57.50/500mg
![N-[4-(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)phenyl]acetamide](/CAS/20180528/GIF/6543-35-7.gif)
6543-35-7
4 suppliers
inquiry

6086-29-9
24 suppliers
$57.50/500mg
122-80-5
260 suppliers
$41.00/25g

6086-29-9
24 suppliers
$57.50/500mg