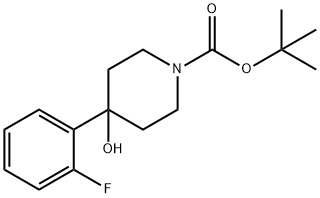
1-N-BOC-4-(2-FLUOROPHENYL)-4-HYDROXYPIPERIDINE synthesis
- Product Name:1-N-BOC-4-(2-FLUOROPHENYL)-4-HYDROXYPIPERIDINE
- CAS Number:403806-35-9
- Molecular formula:C16H22FNO3
- Molecular Weight:295.35
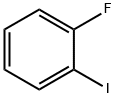
348-52-7
292 suppliers
$6.00/5g

79099-07-3
543 suppliers
$5.00/5g

403806-35-9
16 suppliers
$180.00/1G
Yield:403806-35-9 100%
Reaction Conditions:
Stage #1: 1-Fluoro-2-iodobenzenewith isopropylmagnesium chloride in tetrahydrofuran at 0; for 0.25 h;
Stage #2: N-tert-butyloxycarbonylpiperidin-4-one in tetrahydrofuran at 20;
Stage #3: with water;ammonium chloride in tetrahydrofuran;
Steps:
1b.1 1 st Stage
[0075] A solution of 11.14 g of 2-fluoroiodobenzene in 25 ml of THF was added dropwise at 0° C. to 27.6 ml of a commercially obtained solution of isopropylmagnesium chloride (2 M in THF). After 15 minutes a solution of 10 g (50.2 mmole) of Boc-piperidone XIII in 25 ml of THF was added. The solution was stirred overnight at room temperature. The solution was then hydrolyzed with about 100 ml of ammonium chloride solution and extracted with diethyl ether. The organic phase was dried with sodium sulfate and evaporated to dryness in vacuo. The crude yield was 15.8 g (quantitative). The compound XIV was reacted further in the crude state, and more specifically once with HCl and once with HBr:
References:
US2003/225116,2003,A1 Location in patent:Page 7

1072-85-1
449 suppliers
$5.00/10g

79099-07-3
543 suppliers
$5.00/5g

403806-35-9
16 suppliers
$180.00/1G