1-N-BOC-4-(3-CHLOROPHENYL)-4-HYDROXYPIPERIDINE synthesis
- Product Name:1-N-BOC-4-(3-CHLOROPHENYL)-4-HYDROXYPIPERIDINE
- CAS Number:871112-37-7
- Molecular formula:C16H22ClNO3
- Molecular Weight:311.8
Yield:871112-37-7 78%
Reaction Conditions:
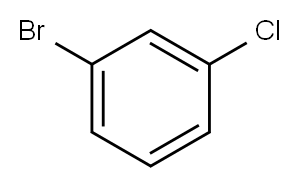
Stage #1: 1-bromo-3-chlorobenzenewith magnesium;iodine in tetrahydrofuran at 0 - 20;Inert atmosphere;
Stage #2: N-tert-butyloxycarbonylpiperidin-4-one in tetrahydrofuran at 20;Inert atmosphere;
Stage #3: with water;ammonium chloride in tetrahydrofuran;Saturated solution;
Steps:
i
tert-Butyl 4-(3-chlorophenyl)-4-(2-(pyrrolidin-1-yl)ethoxy)piperidine-1-carboxylate [amine I]Employed in the Synthesis to Give Example 56 (i) Magnesium (7.2 eq.) was added to tetrahydrofuran (100 ml) under an inert gas atmosphere and a catalytic amount of iodine was added. 3-Bromochlorobenzene (6 eq.) was added in a catalytic amount and the reaction mixture was cooled to 0° C. The 3-bromochlorobenzene solution was added dropwise and the reaction mixture was stirred at room temperature for 3 h. N-Boc-Piperidone (1 eq.) was dissolved in tetrahydrofuran, the solution was added dropwise to the reaction mixture and the mixture was stirred at room temperature for 16 h. After this time, complete reaction of the educts had taken place, via TLC control. Saturated ammonium chloride solution was added to the reaction mixture and the mixture was extracted with ethyl acetate. The organic phase was washed with water and sat. sodium chloride solution, dried over sodium sulfate and concentrated to dryness on a rotary evaporator. The crude product was purified by column chromatography (silica gel, 2% methanol in methylene chloride) to obtain the desired product. Yield: 78%
References:
US2009/264400,2009,A1 Location in patent:Page/Page column 97

79099-07-3
543 suppliers
$5.00/5g
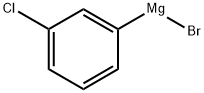
36229-42-2
67 suppliers
$213.00/50ml

871112-37-7
7 suppliers
inquiry