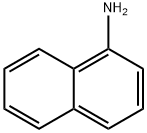
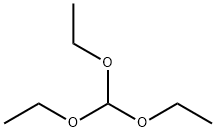
1-naphthalen-1-yltetrazole synthesis
- Product Name:1-naphthalen-1-yltetrazole
- CAS Number:313535-27-2
- Molecular formula:C11H8N4
- Molecular Weight:196.21
Yield:313535-27-2 98%
Reaction Conditions:
with Caswell No. 744A;C113H200N52O40*19Cu(2+)*38C2H3O2(1-) in neat (no solvent) at 120; for 0.5 h;
Steps:
Preparation of 1-substituted-1H-1,2,3,4-tetrazoles
General procedure: A mixture of triethyl orthoformate (1.2 mmol), amine (1.0 mmol), and NaN3 (SafetyNotes: CAUTION Sodium azide is toxic and potentially explosive. Note the comparativelysmall scale of this reaction procedure. Reactions should be carried out in ashielded fume hood with appropriate protective gear. All workers must be thoroughlytrained in the use of this material before undertaking experiments.) (1.0 mmol) in thepresence of 0.04 g (3.6 mol %) Cu(II)/TD was heated and stirred at 120 C under neatconditions for 15-50 min, until TLC (n-hexane:ethyl acetate 9:1) indicated the reactionwas complete. After that, ethyl acetate was added to the reaction mixture and the nanodendrimer catalyst was separated by filtering, rinsed with acetone and then dried overnightto prepare for the next run. The filtrate was concentrated to get a solid product.The crude product was recrystallized from n-hexane:ethyl acetate (9:1). All of the productsof this study were known compounds, identified on the basis of matching theirmelting points with those available in the literature cited in Table 1. For the sake ofcompleteness, representative 1HNMR data are provided below.
References:
Javdan, Samaneh;Moeinpour, Farid;Mohseni-Shahri, Fatemeh S. [Organic Preparations and Procedures International,2022,vol. 54,# 4,p. 346 - 354]
6330-51-4
43 suppliers
$28.60/250mg

313535-27-2
9 suppliers
inquiry