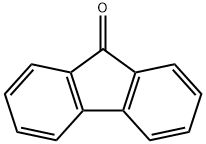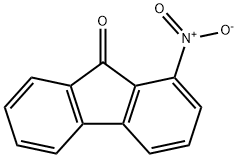
1-Nitro-9H-fluoren-9-one synthesis
- Product Name:1-Nitro-9H-fluoren-9-one
- CAS Number:56825-82-2
- Molecular formula:C13H7NO3
- Molecular Weight:225.2
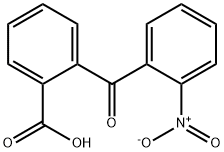
![[1,1'-Biphenyl]-2-carboxylic acid, 3-nitro-](/CAS/20210111/GIF/124391-59-9.gif)
124391-59-9
2 suppliers
inquiry

56825-82-2
7 suppliers
inquiry
Yield:56825-82-2 87%
Reaction Conditions:
with sulfuric acid at 100 - 110;Friedel-Crafts Acylation;
Steps:
1-Nitro-9H-fluoren-9-one (4a)7
3-Nitrobiphenyl-2-carboxylic acid (3a, 1.95 g, 8.0 mmol) was dissolved in sulfuric acid (98%, 6 mL) at room temperature. The brown solution was stirred for 10 minutes at 100 - 110 °C. The resulting black reaction mixture was cooled and poured on ice (25 g.). A gray-green precipitate was formed and S1-3 filtered off. It was washed with aqueous NaHCO3 (5%, 3 mL), H2O (6 x 3 mL), and dried at 60°C. The raw product (1.63 g) was dissolved in CHCl3 (120 mL), filtered through a plug of silica gel. The solvent was evaporated and the residue was recrystallized from MeCN (20 mL) to give the title compound 4a (1.58 g, 7.0 mmol, 87% yield, Rf = 0.2, cyclohexane/CHCl3, 1:3) as yellow rods. 1H NMR (400 MHz, CDCl3) δH 7.75 (d, J = 7.4 Hz, 1HAr, 4-H), 7.70 (d, J = 7.4 Hz, 1HAr, 2-H), 7.63 (t, J= 7.8 Hz, 1HAr, 3-H), 7.60 - 7.52 (m, 3HAr, 5-H, 6-H, 8-H), 7.38 (td, J = 7.3, 1.5 Hz, 1HAr, 7-H). 13C NMR (101 MHz, CDCl3) δC 187.59 (1C, C=O), 146.42 (1CAr, 1-C), 142.28 (1CAr, 5a-C), 135.68(1CArH, 6-C), 135.41 (2C: 1CAr, 4a-C, 1CArH, 3-C), 133.49 (1CAr, 8a-C), 130.63 (1CArH, 7-C), 125.40(1CArH, 8-C), 125.10 (1CAr, 9a-C), 123.77 (1CArH, 4-C), 123.25 (1CArH, 2-C), 120.91 (1CArH, 5-C).
References:
Teodoro, Rodrigo;Scheunemann, Matthias;Wenzel, Barbara;Peters, Dan;Deuther-Conrad, Winnie;Brust, Peter [Bioorganic and Medicinal Chemistry Letters,2018,vol. 28,# 9,p. 1471 - 1475] Location in patent:supporting information
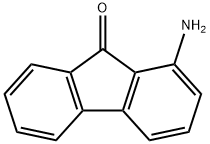
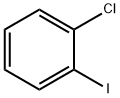
6344-62-3
51 suppliers
$234.00/1g

56825-82-2
7 suppliers
inquiry
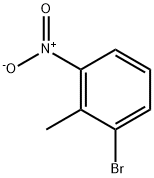
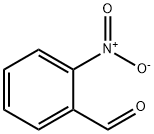
55289-35-5
290 suppliers
$13.00/1g

56825-82-2
7 suppliers
inquiry