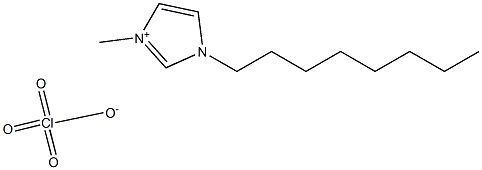
1-octyl-3-MethyliMidazoliuM perchlorate synthesis
- Product Name:1-octyl-3-MethyliMidazoliuM perchlorate
- CAS Number:1400758-87-3
- Molecular formula:C12H23ClN2O4
- Molecular Weight:294.775
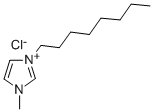
64697-40-1
141 suppliers
$20.39/5g

1400758-87-3
10 suppliers
inquiry
Yield:1400758-87-3 91.9%
Reaction Conditions:
with lithium perchlorate in dichloromethane;water; for 12 h;
Steps:
3 Example 3 Preparation of 1-octyl-3-methylimidazolium acetate ionic liquid:
1.0 mol of 1-octyl-3-methylimidazolium chloride was dissolved in 78 g of water.Solution A was obtained, and 2.0 mol of lithium perchlorate was dissolved in 426 g of water.Solution B was obtained; solution A was mixed with solution B, 841 g of dichloromethane was added, and the reaction was mechanically stirred for 12 hours;The aqueous phase is separated into waste liquid, recovered, and the organic phase is washed with 256 g of pure water each time, and washed 3 times.No precipitation was detected using AgNO3 solution; the organic phase was vacuum-screwed at 80 ° C.193 g of 1-octyl-3-methylimidazolium perchlorate ionic liquid intermediate were obtained in a yield of 91.9%;Add 965g of ethanol, and slowly add a total of 90.1g of potassium acetate in 4 batches with vigorous stirring. During the addition process,Solid solution and precipitation were continued, and the reaction was stirred for 48 hours; stirring was stopped.Freeze to -15 ° C for 12 hours, filter, filter cake recovery; filtrate 80 ° C rotary evaporation; cooled to room temperature,Add 5 volumes of a mixed solution of dichloromethane and 18-crown-6 (crown ether is mixed with dichloromethane in advance,2% by mass of methylene chloride, frozen to -15 ° C for 36 hours, filtered, and the filtrate was steamed at 60 ° C;The 18-crown-6 liquid was removed to obtain a total of 248.3 g of 1-octyl-3-methylimidazolium acetate ionic liquid, and the yield was 90.0%.The purity was determined by high performance liquid chromatography to be 99.1%, K ion residue was ≤100 ppm, and Cl ion residue was ≤5 ppm.
References:
CN110294712,2019,A Location in patent:Paragraph 0048-0050
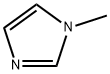
616-47-7
726 suppliers
$10.00/100g

111-83-1
492 suppliers
$12.00/25g

1400758-87-3
10 suppliers
inquiry
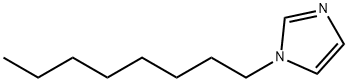
21252-69-7
72 suppliers
$110.00/5g

1400758-87-3
10 suppliers
inquiry