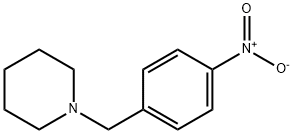
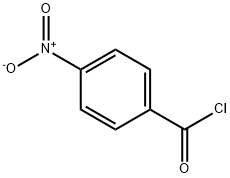
Yield:20857-92-5 99%
Reaction Conditions:
in dichloromethane at 0 - 20; for 0.166667 h;
Steps:
1
Example 1{4-r2-(4-Amino-cvclohexylamino)-9-phenyl-9H-purin-6-ylaminol -phenyl I -piperidin- 1 - yl-methanone[00100] To a solution of piperidine (18.0 g, 211.8 mmol) in dichloromethane (360 mL) at 00C is added 4-nitrobenzoyl chloride (18.6 g, 100 mmol) cautiously in several portions. The reaction mixture is stirred at room temperature for 10 minutes before it is washed with HCl (1%, 2x200 niL) solution and water (300 niL) and dried with Na2SO4. After evaporation of the solvent, (4-nitro-phenyl)-piperidin-l-yl-methanone (23.2 g, 99%) is obtained and used directly in hydrogenation (1. O g of 10% Pd/C in 400 mL of ethanol). After filtration of the catalyst and evaporation of ethanol, (4-amino-phenyl)-piperidin-l-yl- methanone (19.6 g, 96%) is obtained.[00101] A mixture of 2,6-dichloropurine (18.80 g, 100 mmol), 3,4-dihydro-2H-pyran (12.62 g, 150 mmol), p-toluenesulfonic acid monohydrate (1.90 g, 10 mmol) and anhydrous dichlorome thane (200 mL) is stirred at room temperature for 4 hours. After filtration, it is washed with Na2CC^ (10% aqueous, 100 mL), water (100 mL) and dried with Na2SO4. Evaporation of the solvent followed by titration with ethyl acetate (5 mL) and hexanes (60 mL) induces precipitate which upon filtration yields 2,6-dichloro-9-(tetrahydro-pyran-2-yl)- 9H-purine (24.01 g, 88%).[00102] The mixture of 2,6-dichloro-9-(tetrahydro-pyran-2-yl)-9H-purine (5.44 g, 20 mmol), (4-amino-phenyl)-piperidin-l-yl-methanone (4.08 g, 20 mmol), diisopropylethylamine (24 mmol) and ethanol (100 mL) are refluxed for 24 hours. Then ?rans-l,4-cyclohexanediamine (6.84 g, 60 mmol) and diisopropylethylamine (24 mmol) are added and the mixture is refluxed for another 24 hours. The oily residue obtained after evaporation of ethanol is treated with ethyl acetate (250 mL) and water (200 mL). The aqueous phase is extracted with ethyl acetate (2x100 mL) and the combined organic phase dried with Na2SO4. After evaporation, the oily residue obtained is treated withp- toluenesulfonic acid monohydrate (3.80 g, 20 mmol) in methanol (100 mL) at 55°C for 4 hours and the reaction monitored until deprotection is completed. [00103] Diisopropylethylamine is added to neutralize the mixture. The oily residue obtained is subjected to column chromatography (EtOAc: MeOH = 9:1, then Cη2Cl2:Me0η (containing ~7N ammonia) = 9:1) to give 2-(4-amino-cyclohexylamino)-6-[4-(piperidine-l- carbonyl)-phenylamino]-9H-purine (6.50 g, 75%).[00104] A reaction vial containing a mixture of 2-(4-amino-cyclohexylamino)-6-[4-(piperidine-l-carbonyl)-phenylamino]-9H-purine (86.8 mg, 0.2 mmol) prepared as above, copper(I) iodide (38.2 mg, 0.2 mmol) and potassium phosphate (170 mg, 0.8 mmol) is degassed and refilled with dry nitrogen. N,N'-Dimethylethylenediamine (35.3 mg, 43 μL, 0.4 mmol) and iodobenzene (40.8 mg, 0.2 mmol) in DMF (700 μL) are added and the mixture is stirred at 88°C overnight. AcOH-MeOH (1:10, 1.5 mL) is added to neutralize the mixture followed by filtration through a syringe filter. Column chromatography (EtOAc: MeOH = 9:1, then CH2Cl2MeOH (containing ~7N ammonia) = 9: 1) yields {4-[2- (4-amino-cvclohexylamino)-9-phenyl-9H-purin-6-ylaminol-phenyl|-piperidin-l-yl- methanone as a solid; 1H NMR 400 MHz (CD3OD) d 8.03 (s, IH), 7.90-7.95 (m, 2H), 7.75- 7.65 (m, 2H), 7.50-7.42 (m, 2H), 7.38-7.30 (m, 3H), 3.80-3.50 (m, 5H), 2.83-2.73 (m, IH), 2.15-2.05 (m, 2H), 1.95-1.90 (m, 2H), 1.70-1.40 (m, 6H), 1.40-1.20 (m, 4H); MS m/z 511.3 (M+l).
References:
WO2008/94737,2008,A2 Location in patent:Page/Page column 26-28