1-Phenoxy-4-(2-propenyl)benzene synthesis
- Product Name:1-Phenoxy-4-(2-propenyl)benzene
- CAS Number:2653-93-2
- Molecular formula:C15H14O
- Molecular Weight:210.27
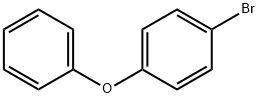
51067-38-0
337 suppliers
$9.00/1g

107-18-6
2 suppliers
$24.60/100ml

2653-93-2
10 suppliers
$252.00/1g
Yield:2653-93-2 40%
Reaction Conditions:
with dicyclohexyl-(2',6'-dimethoxybiphenyl-2-yl)-phosphane;potassium fluoride;tris-(dibenzylideneacetone)dipalladium(0) in toluene at 100; for 24 h;Inert atmosphere;Sealed tube;Reagent/catalyst;
Steps:
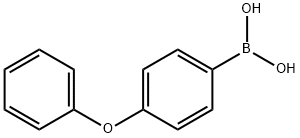
General Procedure B
General procedure: Pd2dba3 (0.025 equiv, 5.7 mg, 0.00625 mmol), SPhos (0.20 equiv, 20.5 mg, 0.050 mmol), 4-phenoxyphenylboronic acid 6a (1.00 equiv, 0.25 mmol) and KF (2.0 equiv, 29.1 mg, 0.50 mmol) in that order, were charged into an oven-dried reaction tube equipped with a magnetic stir bar. The reaction tube was sealed with a rubber septum equipped with screw cap, and then evacuated and backfilled with argon. The evacuation and backfilling sequence was repeated a total of three times. Allylic alcohol (10 equiv, 2.50 mmol) followed by toluene (0.50 mL) were added in that order via syringe through the septum. The reaction tube was then transferred to an oil bath that was preheated to 100 °C. After 24 h, the crude reaction mixture was diluted with ethyl acetate (~1 mL) and passed through a pad of celite (~1 cm) via vacuum filtration. The solution was concentrated under reduced pressure, and the final product was isolated by purification via flash column chromatography.
References:
Leister, Jeffrey;Chao, Darrian;Billingsley, Kelvin L. [Tetrahedron Letters,2021,vol. 66] Location in patent:supporting information

51067-38-0
337 suppliers
$9.00/1g

106-95-6
417 suppliers
$10.00/5g

2653-93-2
10 suppliers
$252.00/1g
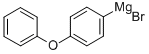
21473-02-9
43 suppliers
$228.00/100ml

106-95-6
417 suppliers
$10.00/5g

2653-93-2
10 suppliers
$252.00/1g
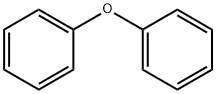
101-84-8
567 suppliers
$5.00/25g

2653-93-2
10 suppliers
$252.00/1g