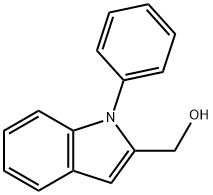
(1-Phenyl-1H-indol-2-yl)-methanol synthesis
- Product Name:(1-Phenyl-1H-indol-2-yl)-methanol
- CAS Number:343238-31-3
- Molecular formula:C15H13NO
- Molecular Weight:223.27
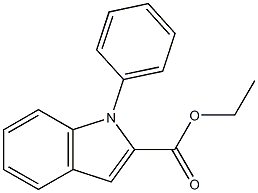
20538-24-3
0 suppliers
inquiry

343238-31-3
4 suppliers
inquiry
Yield:343238-31-3 87.9%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20;Inert atmosphere;
Steps:
General procedure for synthesis of (1-(4-substitutedphenyl)-6-substituted-1H-indol-2-yl)methanol 9a-s.
General procedure: LAH (7 mg, 0.18 mmol) was added portionwise to a stirred solution of 8a-s (0.3 mmol) in 5 mL dry THF. The reaction mixture was stirred for 0.5-4 h at 0 ℃ to room temperature prior to quenching with ethyl acetate. The mixture was filtered through Celite(R). The filtrate was concentrated under reduced pressure and the residue purified by column chromatography (hexane-ethyl acetate, 5:1 - 3:1 v/v) to afford compound 9a-s in 53.1% - 87.9% overall yield.
References:
Wang, Rui;Shi, Hong-Fan;Zhao, Jing-Feng;He, Yan-Ping;Zhang, Hong-Bin;Liu, Jian-Ping [Bioorganic and Medicinal Chemistry Letters,2013,vol. 23,# 6,p. 1760 - 1762] Location in patent:supporting information
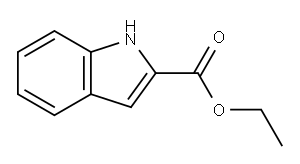
3770-50-1
320 suppliers
$10.00/5g

343238-31-3
4 suppliers
inquiry

100-52-7
942 suppliers
$20.37/250g

343238-31-3
4 suppliers
inquiry
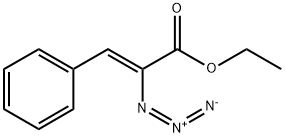
24512-96-7
0 suppliers
inquiry

343238-31-3
4 suppliers
inquiry

108-86-1
498 suppliers
$10.00/5g

343238-31-3
4 suppliers
inquiry