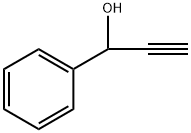
1-phenylbut-2-yne-1,4-diol synthesis
- Product Name:1-phenylbut-2-yne-1,4-diol
- CAS Number:29021-82-7
- Molecular formula:C10H10O2
- Molecular Weight:162.19
Yield:29021-82-7 100%
Reaction Conditions:
Stage #1: Prop-2-ynyl alcoholwith n-butyllithium in tetrahydrofuran;hexane at -40; for 0.333333 h;Inert atmosphere;
Stage #2: benzaldehydewith cerium(III) trichloride in tetrahydrofuran;hexane;Reagent/catalyst;
Steps:
General procedure for the preparation of diols 4-21
General procedure: To a round bottom flask containing the propargylic alcohol (3 mmol) in THF (5 mL) at -40 0C under a dry N 2 atmosphere with stirring, n-butyl lithium (6.6 mmol; 7.2 mL, 0.92 mol/L solution in hexane) was added dropwise. After stirring the solution at the same temperature for 20 min, it was transferred to another round bottomed flask containing a previously prepared mixture of the carbonyl compound (3mmol) and anhydrous CeCl 3 (1.5 mmol) in THF (10 mL). The reaction was monitored by TLC until consumption of the carbonyl compound, quenched with a saturated solution of NH 4 Cl (2 mL) and the phases separated. The aqueous phase was extracted with ethyl acetate (2 x 5 mL) washed with brine, dried with magnesium sulphate, filtered, and the solvents removed using a rotary evaporator under vacuum. The crude was purified by silica gel chromatography using n-hexane/ethyl acetate (1:1) as eluent.
References:
Princival, Jefferson Luiz;Ferreira, Jeiely Gomes [Tetrahedron Letters,2017,vol. 58,# 36,p. 3525 - 3528] Location in patent:supporting information
![Benzenemethanol, α-[3-[(tetrahydro-2H-pyran-2-yl)oxy]-1-propyn-1-yl]-](/CAS/20210305/GIF/52547-82-7.gif)
52547-82-7
0 suppliers
inquiry

29021-82-7
4 suppliers
inquiry
6089-04-9
114 suppliers
$13.43/1gm:

100-52-7
946 suppliers
$20.37/250g

29021-82-7
4 suppliers
inquiry