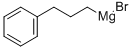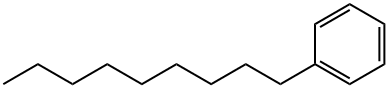
1-Phenylnonane synthesis
- Product Name:1-Phenylnonane
- CAS Number:1081-77-2
- Molecular formula:C15H24
- Molecular Weight:204.35

100-39-0
423 suppliers
$10.00/10 g

38841-98-4
51 suppliers
$91.20/100ml

1081-77-2
83 suppliers
$41.79/10g
Yield: 88 %Chromat.
Reaction Conditions:
with C20H36BrN3NiO in tetrahydrofuran at 20; for 2 h;Kumada Cross-Coupling;Reagent/catalyst;
Steps:
3.7. Typical Procedure for the Kumada Cross-Coupling Reactions in Table 1
General procedure: A vial was charged with alkyl halide substrate (0.2 mmol) and nickel catalyst (3 mol %), and theinternal standard undecane (0.2 mmol) in THF (3 mL). OctylMgCl (0.24 mmol, 2 M in THF) was thenadded dropwise at room temperature. The resulting solution was stirred at room temperature for 2 h.The reaction mixture was then filtered through a plug of silica gel and analyzed by gaschromatography. The response factor of ethyl undecanoate calibrated to the internal standard was usedto calculate product yields.
References:
Yu, Siqi;Wang, Huan;Sledziewski, Jill E.;Madhira, Venkata N.;Takahashi, Cyrus G.;Leon, Michelle K.;Dudkina, Yulia B.;Budnikova, Yulia H.;Vicic, David A. [Molecules,2014,vol. 19,# 9,p. 13603 - 13613]

629-27-6
151 suppliers
$5.00/5g

100-39-0
423 suppliers
$10.00/10 g

1081-77-2
83 suppliers
$41.79/10g
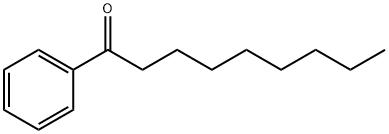
6008-36-2
51 suppliers
$73.09/10g

1081-77-2
83 suppliers
$41.79/10g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1081-77-2
83 suppliers
$41.79/10g