
[1-(Phenylsulphonyl)piperidin-3-yl]methanol synthesis
- Product Name:[1-(Phenylsulphonyl)piperidin-3-yl]methanol
- CAS Number:346691-49-4
- Molecular formula:C12H17NO3S
- Molecular Weight:255.33
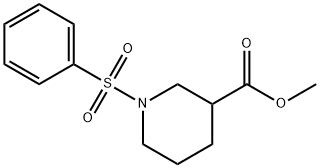
1040877-93-7
4 suppliers
inquiry
![[1-(Phenylsulphonyl)piperidin-3-yl]methanol](/CAS/20140601/GIF/CB41262609.gif)
346691-49-4
9 suppliers
$181.00/500mg
Yield:346691-49-4 91.4%
Reaction Conditions:
Stage #1: 1-benzenesulfonyl-piperidine-3-carboxylic acid methyl esterwith lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20;
Stage #2: with potassium hydroxide in tetrahydrofuran;water at 0;
Steps:
1.1.C
The methyl ester (51 g, 180.2 mmol, 1 equiv.) from above, was dissolved in dry THF (500 mL) and transferred into pressure equalizing funnel. Lithium aluminum hydride (8.2 Ig, 216.2 mmol, 1.2 equiv.) was suspended in dry THF (100 mL) in a round bottomed flask (1 L). The ester from dropping funnel was added dropwise at 0 0C to 5 0C. After the complete addition, the reaction mixture was brought to room temperature and stirred. The reaction was monitored by TLC till completion. The
References:
WO2008/87654,2008,A2 Location in patent:Page/Page column 25-26