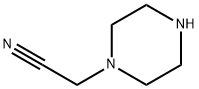
1-Piperazineacetonitrile(6CI,9CI) synthesis
- Product Name:1-Piperazineacetonitrile(6CI,9CI)
- CAS Number:58619-56-0
- Molecular formula:C6H11N3
- Molecular Weight:125.17
Yield:58619-56-0 72%
Reaction Conditions:
Stage #1: chloroacetonitrile;1-t-Butoxycarbonylpiperazinewith sodium carbonate in ethanol at 100; for 5 h;Microwave irradiation;
Stage #2: with hydrogenchloride in 1,4-dioxane;methanol at 20; for 20 h;
Stage #3: with ammonium hydroxide in methanol;water; for 1 h;
Steps:
1.2.4.2.4.1 2.4.1 General procedure B
General procedure: N-Boc-piperazine or N-Boc-homopiperazine (1.0 eq) was dissolved in absolute EtOH and subsequently 2-chloroacetonitrile (1.2 eq) and Na2CO3 (2.0 eq) were added. The reaction mixture was challenged by MW irradiation for 5 hours at 100 °C. The solution was concentrated, diluted with H2O and three times washed with DCM (3x 50 mL). The organic phases were collected, dried over Na2SO4, filtered, concentrated and used into next step without any further purification. The residue was dissolved in MeOH (30 mL) and 1 M solution of HCl in dioxane (15 mL) was added. The mixture was stirred at RT for 20 hours. The result was concentrated and dried under reduced pressure. The residue was again dissolved in MeOH (30 mL) and 25 % solution of ammonium hydroxide (NH4OH) in water (15 mL) was added. After another 1 hour of stirring the reaction mixture was concentrated and directly purified by column chromatography using mobile phase DCM/MeOH/NH4OH (6:1:0.1) to give crude products.
References:
WO2020/237079,2020,A1 Location in patent:Page/Page column 38; 41; 43-44
676341-78-9
0 suppliers
inquiry

58619-56-0
17 suppliers
inquiry