1-PIPERIDIN-4-YL-2,3-DIHYDRO-1H-INDOLE synthesis
- Product Name:1-PIPERIDIN-4-YL-2,3-DIHYDRO-1H-INDOLE
- CAS Number:181525-34-8
- Molecular formula:C13H18N2
- Molecular Weight:202.3
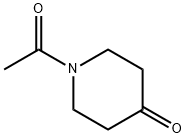
400828-91-3
18 suppliers
$205.00/1g

181525-34-8
11 suppliers
inquiry
Yield:181525-34-8 84%
Reaction Conditions:
with trifluoroacetic acid in dichloromethane at 0 - 20; for 2 h;
Steps:
1.1 Step 1
General procedure: A solution of N-Boc intermediate (1.00 equiv) in CH2C12 (0.25-0.30M) was cooled to 0 °C, and then TFA (6-30 equiv) was added over several minutes. Upon completion of addition, the ice-bath was removed and the reaction was allowed to warm to room temperature and monitored by TLC (EtOAc:Hexanes). After 2 hours, the reaction was complete. The reaction was concentrated in vacuo, followed by the addition of EtOAc, which was consequently removed in vacuo. The oil residue was then dissolved in EtOAc and was stirred as saturated NaHCO3 (aq.) was added until the aqueous layer remained basic. The layers were separated, and the aqueous layer was extracted with EtOAc until UV activity in the aqueous layer was minimal (3-8x). The EtOAc layers were combined, washed with brine, dried with Mg504, filtered, and concentrated in vacuo to provide the piperidine intermediate.
References:
WO2017/96323,2017,A1 Location in patent:Page/Page column 102; 103

79099-07-3
543 suppliers
$5.00/5g

181525-34-8
11 suppliers
inquiry
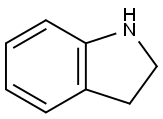
496-15-1
425 suppliers
$8.00/10g

181525-34-8
11 suppliers
inquiry