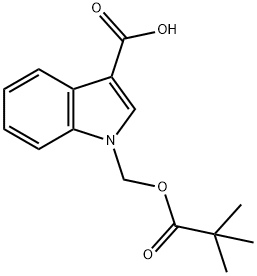
1-(PIVALOYLOXYMETHYL)-1H-INDOLE-3-CARBOXYLIC ACID synthesis
- Product Name:1-(PIVALOYLOXYMETHYL)-1H-INDOLE-3-CARBOXYLIC ACID
- CAS Number:739365-21-0
- Molecular formula:C15H17NO4
- Molecular Weight:275.3
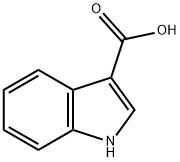
771-50-6
579 suppliers
$5.00/100mg
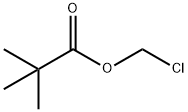
18997-19-8
311 suppliers
$5.00/5g

739365-21-0
1 suppliers
inquiry
Yield:739365-21-0 79%
Reaction Conditions:
Stage #1: 1H-indole-3-carboxylic acidwith sodium hydride in DMF (N,N-dimethyl-formamide) at 0; for 0.5 h;
Stage #2: Chloromethyl pivalate in DMF (N,N-dimethyl-formamide) at 0 - 20; for 2 h;
Steps:
53
(Intermediate Example 53) 1-(2,2-Dimethylpropionyloxymethyl)-1H-indole-3-carboxylic acid Sodium hydride (218 mg) was added to a solution of 1H-indole-3-carboxylic acid (400 mg) in N,N-dimethylformamide (4 ml) with ice cooling, and the mixture was stirred for 30 minutes. Chloromethyl 2,2-dimethylpropionate (373 mg) was added thereto, and the mixture was warmed to room temperature and stirred for 2 hours. Water was added thereto, and the aqueous phase was washed with ether. The aqueous phase was acidified by 2 N hydrochloric acid and extracted with ether. The organic phase was washed with a saturated saline solution and dried over sodium sulfate anhydrous. The product was concentrated under reduced pressure to give the title compound (540 mg, Y.: 79%) as orange crystals. ESI/MS (m/z): 276 (M+H)+, 274 (M-H)-.
References:
EP1595866,2005,A1 Location in patent:Page/Page column 22