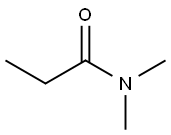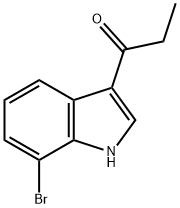
1-Propanone, 1-(7-broMo-1H-indol-3-yl)- synthesis
- Product Name:1-Propanone, 1-(7-broMo-1H-indol-3-yl)-
- CAS Number:179473-61-1
- Molecular formula:C11H10BrNO
- Molecular Weight:252.11
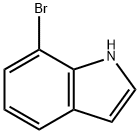
51417-51-7
368 suppliers
$12.00/1g
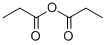
123-62-6
302 suppliers
$17.00/25g

179473-61-1
2 suppliers
inquiry
Yield:179473-61-1 89 %
Reaction Conditions:
with boron trifluoride diethyl etherate in dichloromethane at 20;Inert atmosphere;regioselective reaction;
Steps:
3.1. General Procedure for the Synthesis of Product (3aa-3jd)
General procedure: A mixture of indole 1 (0.5 mmol), anhydride 2 (0.6 mmol), and BF3Et2O (BF3 46.5%)(0.5 mmol, 64 L) in DCM was stirred at room temperature for the desired time. After thereaction was completed, saturated sodium bicarbonate (10 mL) was added. The reactionmixture was stirred for 5 min and then extracted with ethyl acetate (3 10 mL). Theorganic layer was dried over anhydrous Na2SO4 and, after evaporation of the solventunder reduced pressure, the residue was purified by column chromatography on silica gelusing petroleum ether/EtOAc (6:1 to 2:1) as the eluents to give product 3.
References:
Zheng, Yunyun;Li, Jiuling;Wei, Kai [Molecules,2022,vol. 27,# 23,art. no. 8281]