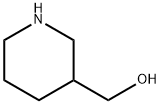
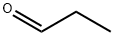(1-Propylpiperidin-3-yl)methanol synthesis
- Product Name:(1-Propylpiperidin-3-yl)methanol
- CAS Number:102450-20-4
- Molecular formula:C9H19NO
- Molecular Weight:157.2533
Yield:-
Reaction Conditions:
with sodium tris(acetoxy)borohydride;acetic acid in chloroform at 0 - 20; for 21 h;
Steps:
B.19 Reference Example B19
Reference Example B19 To a solution of 3-piperidine methanol (1.0 g) and propionaldehyde (1.2 mL) in chloroform (10 mL) was added successively sodium triacetoxyborohydride (3.54 g) and acetic acid (1.0 mL) under ice-cooling and the mixture was stirred at room temperature for 21 hours. The reaction mixture was diluted with chloroform and acidified (pH 10) with an aqueous saturated sodium hydrogencarbonate solution under stirring. The aqueous layer was saturated with potassium carbonate powder and extracted with chloroform (x 2). The extract was dried over sodium sulfate and concentrated to give (1-propyl-piperidin-3-yl)methanol (1.82 g) as a crude product. MS(APCI)m/z; 158 [M+H]+
References:
TANABE SEIYAKU CO., LTD. EP1772454, 2007, A1 Location in patent:Page/Page column 72-73