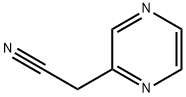
1-(pyrazin-2-yl)cyclopropanecarbonitrile synthesis
- Product Name:1-(pyrazin-2-yl)cyclopropanecarbonitrile
- CAS Number:1159734-50-5
- Molecular formula:C8H7N3
- Molecular Weight:145.16
Yield:1159734-50-5 31%
Reaction Conditions:
with sodium hydride in N,N-dimethyl-formamide at 20; for 18 h;
Steps:
1 Step 1 : 1-(Pyrazin-2-yl)cyclopropanecarbonitrile
Step 1 : 1-(Pyrazin-2-yl)cyclopropanecarbonitrile To a suspension of sodium hydride (0.386 g, 16.1 mmol) in anhydrous N,N- dimethylformamide (5 mL) was added dropwise a solution of 2-(pyrazin-2-yl)acetonitrile (0.55 g, 4.6 mmol) and 1 ,2-dibromoethane (2.25 g, 1 1.9 mmol) in anhydrous N,N- dimethylformamide (8 mL) over a period of 10 min at room temperature. The mixture was stirred for 18 h, then was quenched with water and extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate, filtered, and concentrated under reduced pressure. The crude material was purified via column chromatography (100-200 mesh silica gel, 100% dichloromethane) to afford 1-(pyrazin- 2-yl)cyclopropanecarbonitrile (210 mg, 31%).
References:
WO2013/150416,2013,A1 Location in patent:Page/Page column 107

4949-13-7
122 suppliers
$20.00/1g
5500-21-0
311 suppliers
$16.00/5mL

1159734-50-5
18 suppliers
inquiry