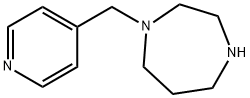
1-(pyridin-4-ylmethyl)-1,4-diazepane synthesis
- Product Name:1-(pyridin-4-ylmethyl)-1,4-diazepane
- CAS Number:199938-13-1
- Molecular formula:C11H17N3
- Molecular Weight:191.27
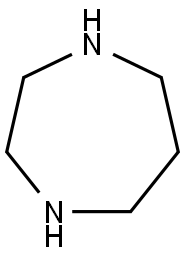
505-66-8
391 suppliers
$5.00/1g
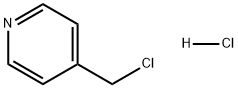
1822-51-1
325 suppliers
$13.00/25g

199938-13-1
11 suppliers
$682.00/0.5G
Yield:199938-13-1 72%
Reaction Conditions:
Stage #1: 1,4-Diazacycloheptane;4-picolylchloride hydrochloridewith ethanol;acetyl chloride in ethanol; for 17.5 h;Heating / reflux;
Stage #2: with potassium hydroxide in dichloromethane;water;
Steps:
[00324] A solution of acetyl chloride (6.34 mL, 0.084 mol, 4 equiv) dissolved in anhydrous EtOH (50 mL) was stirred for 0.5 h and added to a solution of homopiperazine (10.4 g, 0.1 mol, 5 equiv) in EtOH (250 mL). The reaction mixture was heated to reflux for 1 h, cooled to 25° C. and a solution of 4-picolyl chloride hydrochloride 93.44 g, 0.021 mol) in EtOH (40 mL) was added. The reaction mixture was heated to reflux for 16 h, cooled to 25° C. and the solvent was removed under vacuum. The residue was diluted with CH2Cl2 (300 mL) and was washed with 2N KOH (1×300 mL). The aqueous layer was extracted with CH2Cl2 (1×300 mL) and the organic phase was washed with 2N KOH (150 mL), dried (MgSO4) and concentrated. Chromatography (SiO2, 5% H2O-5% NH4OH-1PrOH) afforded the desired product (2.88 g, 4.01 g theoretical, 72%) as a yellow oil. TLC Rf 0.45 (5% H2O-5% NH4OH-1PrOH): 1H NMR (CDCl3, 300 MHz) δ 8.77 (d, J=5.9 Hz, 2H), 7.53 (d, J=5.7 Hz, 2H), 3.91 (s, 2H), 3,19 (m, 4H), 2.92 (m, 4H), 2.04 (m, 2H).
References:
US6686353,2004,B1 Location in patent:Page/Page column 107-108