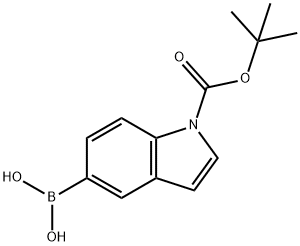
1-(TERT-BUTOXYCARBONYL)-1H-INDOL-5-YLBORONIC ACID synthesis
- Product Name:1-(TERT-BUTOXYCARBONYL)-1H-INDOL-5-YLBORONIC ACID
- CAS Number:317830-84-5
- Molecular formula:C13H16BNO4
- Molecular Weight:261.08
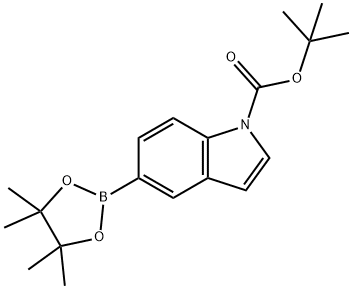
777061-36-6
92 suppliers
$45.00/100mg

317830-84-5
39 suppliers
$120.00/1g
Yield:317830-84-5 54%
Reaction Conditions:
with sodium periodate;ammonium acetate;water in acetone at 23; for 24 h;
Steps:
59
Example 59. N-Boc-indole-5-boronic acid (S49)To iV-Boc-indole-5-boronic acid pinacol ester (172 mg, 0.500 mmol, 1.00 equiv) in acetone/H2O (5.0 mL/5.0 mL) at 23 0C was added NH4OAc (193 mg, 2.50 mmol, 5.00 equiv) and NaIO4 (535 mg, 2.00 mmol, 4.00 equiv). After stirring for 24 hr at 23 0C, the reaction mixture was concentrated in vacuo to remove acetone. To the residual solution was added EtOAc (5 mL) and the phases were separated. The aqueous phase was extracted with EtOAc (2 x 5 mL). The combined organic phases are washed with brine (10 mL) and dried (Na2SO4). The filtrate was concentrated in vacuo and the residue was purified by chromatography on silica gel eluting with hexanes/EtOAc 1:1 (v/v) to afford 70.0 mg of the title compound as a colorless solid (54% yield).R/= 0.50 (hexanes/EtOAc 1:1 (v/v)). NMR Spectroscopy: 1H NMR (500 MHz, CDCl3, 23 0C, δ): 8.56 (s, IH), 8.31-8.23 (m, 2H), 7.67 (d, /= 3.0 Hz, IH), 6.75 (d, /= 3.5 Hz, IH), 1.72 (s, 9H). 13C NMR (125 MHz, CDCl3, 23 0C, δ): 149.71, 138.05, 131.31, 130.42, 129.41, 126.19, 124.31 (br), 114.69, 107.76, 83.91, 28.21. Mass Spectrometry: HRMS-FIA (m/z): Calcd for [M + Na]+, 284.10646. Found, 284.10767.
References:
WO2010/59943,2010,A2 Location in patent:Page/Page column 85
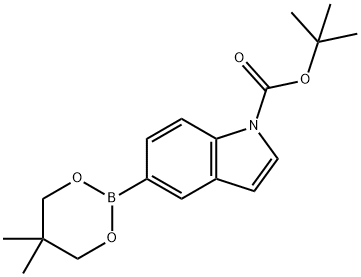
1167418-11-2
0 suppliers
inquiry

317830-84-5
39 suppliers
$120.00/1g
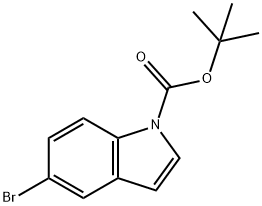
182344-70-3
97 suppliers
$9.00/250mg

317830-84-5
39 suppliers
$120.00/1g
10075-50-0
692 suppliers
$5.00/10g

317830-84-5
39 suppliers
$120.00/1g