
1-(TETRAHYDRO-2H-PYRAN-4-YL)PIPERIDIN-4-AMINE synthesis
- Product Name:1-(TETRAHYDRO-2H-PYRAN-4-YL)PIPERIDIN-4-AMINE
- CAS Number:794471-13-9
- Molecular formula:C10H20N2O
- Molecular Weight:184.28

873537-63-4
9 suppliers
inquiry

794471-13-9
28 suppliers
$45.00/25mg
Yield:794471-13-9 92%
Reaction Conditions:
Stage #1: tert-butyl (1-(tetrahydro-2H-pyran-4-yl)piperidin-4-yl)carbamatewith hydrogenchloride in 1,4-dioxane;methanol at 20;
Stage #2: with sodium hydroxide in dichloromethane;water;
Steps:
9.b
9.2 g (0.0323 mol, 1 eq) of 1 ,1-dimethylethyl [1-(tetrahydro-2/7-pyran-4-yl)-4- piperidinyl]carbamate, 65 mL (0.258 mol, 8 eq) of 4 M HCI in dioxane, and enough methanol to achieve dissolution were combined and stirred at ambient temperature overnight. The reaction mixture was then evaporated. Dichloromethane and 1 M NaOH were added. Sodium chloride was then added to saturate the aqueous layer. The aqueous layer was extracted with dichloromethane twice. The organic layers were combined, dried with sodium sulfate, and evaporated to yield 5.5 g (92% yield) of 1- (tetrahydro-2/-/-pyran-4-yl)-4-piperidinamine as a low melting white solid. 1H-NMR (400 MHz, DMSO-Qf6): δ 3.86 (m, 2H), 3.24 (m, 2H), 2.77 (m, 2H), 2.46 (m, 1H), 2.35 (m, 1 H), EPO
References:
WO2007/36711,2007,A1 Location in patent:Page/Page column 44-45
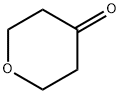
29943-42-8
421 suppliers
$5.00/1g
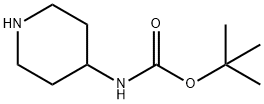
73874-95-0
449 suppliers
$10.00/250mg

794471-13-9
28 suppliers
$45.00/25mg