
1-(trifluoroacetyl)piperidin-4-one synthesis
- Product Name:1-(trifluoroacetyl)piperidin-4-one
- CAS Number:65220-86-2
- Molecular formula:C7H8F3NO2
- Molecular Weight:195.14
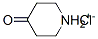
41979-39-9
0 suppliers
$15.00/10mg
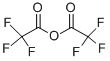
407-25-0
470 suppliers
$9.00/1g

65220-86-2
31 suppliers
$157.00/250mg
Yield:65220-86-2 100%
Reaction Conditions:
Stage #1: 4-piperidone hydrochloridewith triethylamine in dichloromethane; for 0.166667 h;
Stage #2: trifluoroacetic anhydride in dichloromethane at 0 - 20; for 2 h;
Steps:
N-(4-Fluorobenzyl)-N-(piperidin-4-yl)-N'-(4-isobutoxybenzyl)carbamide Hydrochloride; 4-Piperidone hydrochloride monohydrate (4.0 g, 26.0 mmol) was dissolved in dichloromethane (130 mL). After addition of triethylamine (8.66 g, 85.8 mmol) the mixture was stirred for 10 min and then cooled to 0° C. Trifluoroacetic anhydride (12.0 g, 57.2 mmol) was added dropwise under stirring. After 2 hours at room temperature, the reaction was stopped by addition of water (100 mL). The aqueous phase was extracted with dichloromethane (2×100 mL). The combined organic phases were dried over Na2SO4, filtered and concentrated to give 1-trifluoroacetyl-4-piperidone (5.07 g, 100%). 4-Fluorobenzylamine (3.14 g, 25.9 mmol) and 1-trifluoroacetyl-4-piperidone (5.07 g, 25.9 mmol) were added to a solution of methanol adjusted to pH 5 with acetic acid (150 mL). The reaction mixture was stirred for 5 min and NaBH3CN (2.46 g, 38.9 mmol) was added slowly under stirring. After 20 hours at room temperature the reaction was concentrated. 2M aqueous NaOH (100 mL) was added and extracted with dichloromethane (2×100 mL). The combined organic phases were dried over Na2SO4, filtered and concentrated to give N-(4-fluorobenzyl)-4-amino-1-trifluoroacetylpiperidine (2.91 g, 37%).
References:
US2009/82342,2009,A1 Location in patent:Page/Page column 15
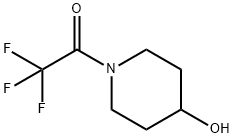
93343-02-3
7 suppliers
inquiry

65220-86-2
31 suppliers
$157.00/250mg
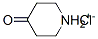
41979-39-9
0 suppliers
$15.00/10mg

65220-86-2
31 suppliers
$157.00/250mg
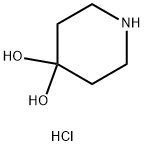
40064-34-4
0 suppliers
$10.00/5g

65220-86-2
31 suppliers
$157.00/250mg
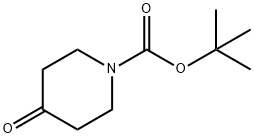
79099-07-3
0 suppliers
$5.00/5g

65220-86-2
31 suppliers
$157.00/250mg