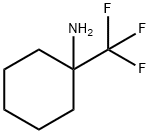
1-(trifluoroMethyl)cyclohexanaMine hydrochloride synthesis
- Product Name:1-(trifluoroMethyl)cyclohexanaMine hydrochloride
- CAS Number:134424-35-4
- Molecular formula:C7H12F3N
- Molecular Weight:167.17
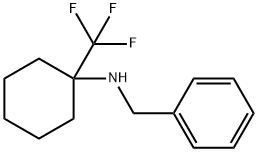
1100246-29-4
4 suppliers
inquiry

134424-35-4
11 suppliers
inquiry
Yield:134424-35-4 81%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in methanol; under 37503.8 Torr;
Steps:
General procedure for the preparation ofgem-trifluoromethylamines 4
A suspension of ketone 1 (0.162 mol), benzylamine (18.6 mL, 0.170 mol), and dry powdered magnesium sulphate (97.2 g, 0.81 mol) in dichloromethane (500 mL) was stirred at room temperature for 96 h. The salt was filtered off; the solvent was removed under reduced pressure and the residue was dried under vacuum for 6 hrs. Upon drying the crude intermediate imine 2 was dissolved in acetonitrile (300 mL). Trifluoroacetic acid (16.0 mL, 0.209 mmol), NaHF2 (10.2 g, 0.131 mol), and DMF (37 mL) were added to the solution of imine 2 at 0 °C. The reaction mixture was stirred for 5 min. Me3SiCF3 (38.6 mL, 0.243 mol) was added to the reaction mixture and the mixture was stirred at ambient temperature for 12 h. Then, saturated aqueous Na2CO3 (60 mL) was added and the mixture was stirred for 5 min. The mixture was diluted with water (500 mL), and extracted with ethyl acetate (3 x 300 mL). The combined organic layers were washed with water and brine, dried over Na2SO4 and evaporated under reduced pressure. The residue was dissolved in 2M HCl/MeOH and solvent was evaporated. The resulting hydrochloride salt was crystallized from CH3CN to provide amine 3 which was used in the next stage. Amine 3 was dissolved in MeOH (20 mL) and the solution was stirred overnight with Pd/C (10% mol) under H2 atmosphere at 50 bar. Then the catalyst was filtered off and the solvent was removed under reduced pressure. The crude product was crystallized from CH3CN, filtered and washed with diethyl ether to give the final product 4 in the form of hydrochloride.
References:
Radchenko, Dmytro S.;Michurin, Oleg M.;Chernykh, Anton V.;Lukin, Oleg;Mykhailiuk, Pavel K. [Tetrahedron Letters,2013,vol. 54,# 14,p. 1897 - 1898] Location in patent:supporting information
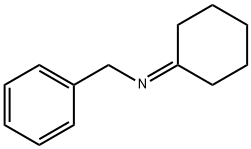
4471-09-4
0 suppliers
inquiry

134424-35-4
11 suppliers
inquiry

108-94-1
531 suppliers
$12.00/50g

134424-35-4
11 suppliers
inquiry