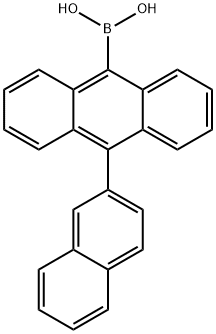
10-(2-Naphthyl)anthracene-9-boronic acid synthesis
- Product Name:10-(2-Naphthyl)anthracene-9-boronic acid
- CAS Number:597554-03-5
- Molecular formula:C24H17BO2
- Molecular Weight:348.2
474688-73-8
228 suppliers
$15.00/1g
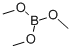
121-43-7
374 suppliers
$13.00/25mL

7732-18-5
485 suppliers
$12.69/100ml

597554-03-5
221 suppliers
$13.00/250mg
Yield:597554-03-5 80%
Reaction Conditions:
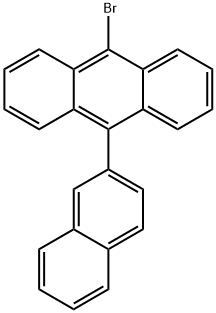
Stage #1:9-bromo-10-(2-naphtyl)-anthracene with n-butyllithium in tetrahydrofuran;hexane at -78; for 3 h;Inert atmosphere;
Stage #2:Trimethyl borate in tetrahydrofuran;hexane at 20;
Stage #3:water with hydrogenchloride in tetrahydrofuran;hexane for 24 h;
Steps:
10-(Naphthalen-2-yl)anthracen-9-yl)boronic acid (Compound 2
10-(Naphthalen-2-yl)anthracen-9-yl)boronic acid (Compound 2
To a solution of 9-bromo-10-(naphthalen-2-yl)anthracene (Compound 1) (3.84 g, 10 mmol) in THF (50 ml) was added n-BuLi solution (1.6 M in hexanes, 7.5 ml) at -78° C. under argon.
The mixture was stirred for about 3 hours at -78° C., then freshly distilled trimethylborate (2.5 ml) was added.
The whole was then warmed up to room temperature overnight, then 5% HCl aqueous solution (100 ml) was added and stirred for about one day.
Filtration gave a white solid (1.7 g), and the filtrate was concentrated and gave a yellow solid, which was washed with hexanes (100 ml*2) which gave a light yellow solid (1.8 g).
Total amount of the product was 3.6 g, in 80% yield.
References:
NITTO DENKO CORPORATION;Zheng, Shijun US9923145, 2018, B2 Location in patent:Page/Page column 34; 35

474688-73-8
228 suppliers
$15.00/1g

597554-03-5
221 suppliers
$13.00/250mg

474688-73-8
228 suppliers
$15.00/1g
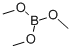
121-43-7
374 suppliers
$13.00/25mL

597554-03-5
221 suppliers
$13.00/250mg

474688-73-8
228 suppliers
$15.00/1g

5419-55-6
386 suppliers
$12.00/5g

597554-03-5
221 suppliers
$13.00/250mg

474688-73-8
228 suppliers
$15.00/1g
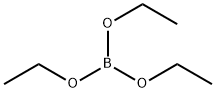
150-46-9
201 suppliers
$12.00/25mL

597554-03-5
221 suppliers
$13.00/250mg