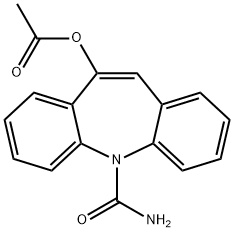
10-Acetoxy-5H-dibenz[b,f]azepine-5-carboxaMide synthesis
- Product Name:10-Acetoxy-5H-dibenz[b,f]azepine-5-carboxaMide
- CAS Number:952740-00-0
- Molecular formula:C17H14N2O3
- Molecular Weight:294.3
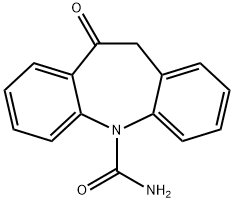
28721-07-5
555 suppliers
$5.00/50mg

108-24-7
5 suppliers
$14.00/250ML
![10-Acetoxy-5H-dibenz[b,f]azepine-5-carboxaMide](/CAS/20150408/GIF/952740-00-0.gif)
952740-00-0
29 suppliers
$155.00/10mg
Yield:952740-00-0 88%
Reaction Conditions:
with pyridine;dmap in dichloromethane at 20; for 4.16 h;
Steps:
Preparation of enol acetate (R = methyl); To a suspension of oxcarbazepine (69.3 g, 0.275 mol), DMAP (1.025 g) and acetic anhydride (38.07 g) in dichloromethane (700 mL) was added drop wise a solution of 30.1 g pyridine in 50 mL dichloromethane at room temperature. The addition completed in 10 min. After stirring at room temperature for 75 min, the system became clear. Three hours after the addition, the system became cloudy again. The suspension was then stirred at room temperature for one more hour and washed with 2 x 400 mL
References:
WO2007/117166,2007,A1 Location in patent:Page/Page column 12-13
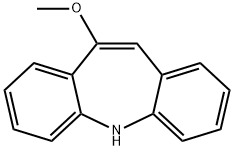
4698-11-7
243 suppliers
$5.00/1g
![10-Acetoxy-5H-dibenz[b,f]azepine-5-carboxaMide](/CAS/20150408/GIF/952740-00-0.gif)
952740-00-0
29 suppliers
$155.00/10mg