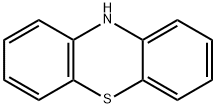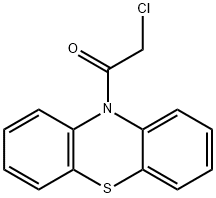
10-(chloroacetyl)-10H-phenothiazine synthesis
- Product Name:10-(chloroacetyl)-10H-phenothiazine
- CAS Number:786-50-5
- Molecular formula:C14H10ClNOS
- Molecular Weight:275.75
Yield:786-50-5 95%
Reaction Conditions:
in toluene at 0 - 80; for 12 h;
Steps:
4.1.1 2-chloro-1-(10H-phenothiazin-10-yl)ethanone (2a)
General procedure: 4.1.1
2-chloro-1-(10H-phenothiazin-10-yl)ethanone (2a)
To the solution of 10H-phenothiazine 1 (5.0 g, 25.11 mmol) in toluene (75 mL) was cooled to 0 °C.
To the above solution chloroacetyl chloride (3.0 mL, 37.6 mmol) was added and reaction mixture was heated at 80 °C for 12 h.
The reaction mixture was allowed cool to room temperature, concentrated under reduced pressure (<45 °C) and to the crude material water (50 mL) was added and extracted with dichloromethane (2 * 50 mL).
Organic layer was dried over anhydrous Na2SO4 and the solvent was removed to get compound 2a as off white solid. Yield: 6.56 g, 95%; m.p: 114-115 °C; FTIR (ATR, cm-1): 2998, 2950, 1670, 1586, 1476, 1459, 1442, 1401, 1339, 1275, 1248, 1193, 1170, 1123, 782, 751, 647, 615; 1H NMR (400 MHz, CDCl3) δ (ppm):7.52 (s, 2H, Ar-H), 7.40 (s, 2H, Ar-H), 7.29 (d, J = 5.7 Hz, 2H, Ar-H), 7.21 (d, J = 6.3 Hz, 2H, Ar-H), 4.12 (s, 2H, CH2); 13C NMR (100 MHz, CDCl3) δ (ppm): 165.5, 137.9, 133.1, 128.1, 127.4, 127.3, 126.5, 41.8; ESI-MS (m/z) = 276.1 (M + 1); calculated for C14H10ClNOS; C, 60.98; H, 3.66; N, 5.08; S, 11.63. Found: C, 60.87; H, 3.68; N, 5.10; S, 11.65.
References:
Ramprasad, Jurupula;Nayak, Nagabhushana;Dalimba, Udayakumar [European Journal of Medicinal Chemistry,2015,vol. 106,p. 75 - 84]