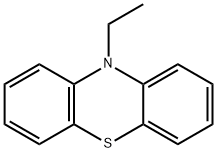
10-ethyl-10H-phenothiazine synthesis
- Product Name:10-ethyl-10H-phenothiazine
- CAS Number:1637-16-7
- Molecular formula:C14H13NS
- Molecular Weight:227.32
Yield: 99%
Reaction Conditions:
Stage #1:10H-phenothiazine with sodium hydride in tetrahydrofuran;mineral oil at 0; for 1 h;Inert atmosphere;
Stage #2:ethyl bromide in tetrahydrofuran;mineral oil at 20; for 12.5 h;Inert atmosphere;
Steps:
10-Ethylphenothiazine, PTZEt
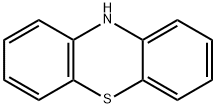
10-Ethylphenothiazine was prepared by modification of two published procedures10, 11.Phenothiazine (6.0 g, 30 mmol) was dissolved in dry THF (70 ml) at 0 °C in a 200 mL two neckround bottom flask. The solution was purged with argon for 15 minutes. Solid sodium hydrideNaH, 60% dispersion in mineral oil (1.8 g, 45 mmol) was added in small portions to thephenothiazine solution while stirring and maintaining argon atmosphere. Stirring was continuedfor approx. 1 h until the solution had completely turned yellow. 1-Bromo ethane (5.0 g, 45mmol) dissolved in 20 mL of dry THF was added drop wise for a period of 30 min. The reactionmixture was kept stirring at RT for 12 hours, and by then the solution had turned clear. 100 mLof distilled water was added and extracted with 2 x 50 mL of diethyl ether. The aqueous phasewas washed with 50 mL dichloromethane. The combined organic phase was dried with NaHCO3,and concentrated to obtain a white solid (6.7 g, yield 99%). 1H NMR (400MHz, RT, CDCl3): δ(ppm) 7.12-7.16 (m, 4H), 6.86-9.92 (m, 4H), 3.94 (q, J = 6.96, 2H); 1.42 (t, J = 6.96, 3H). 1H NMR (500MHz, RT, CDCl3): δ (ppm) 7.12-7.16 (m, 4H); 6.86-9.92 (m, 4H), 3.94 (q, J =6.96, 2H); 1.42 (t, J = 6.96, 3H).
References:
Kosgei, Gilbert K.;Livshits, Maksim Y.;Canterbury, Theodore R.;Rack, Jeffery J.;Brewer, Karen J. [Inorganica Chimica Acta,2017,vol. 454,p. 67 - 70] Location in patent:supporting information
1628-29-1
51 suppliers
$6.00/100mg

1637-16-7
17 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1637-16-7
17 suppliers
inquiry