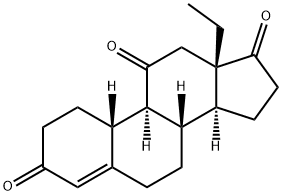
100071-90-7 synthesis
- Product Name:100071-90-7
- CAS Number:100071-90-7
- Molecular formula:C19H24O3
- Molecular Weight:300.39

53067-82-6
39 suppliers
inquiry

100071-90-7
9 suppliers
inquiry
Yield:100071-90-7 40.21 g
Reaction Conditions:
with sulfur trioxide pyridine complex;dimethyl sulfoxide;triethylamine at 25 - 35; for 3 h;Inert atmosphere;
Steps:
1 EXAMPLE 1 Preparation of 18-methyl-estra-4-en-3,11,17-trione, compound (III)
50 g of compound (II) are dissolved in 125 mL DMSO; 229 mL TEA are then added. Keeping the temperature between 25 °C and 35 °C, 79 g of PySO3 dissolved in 225 mL DMSO are added. Exothermy is observed during the addition. Stirring is continued for 3 hours at 25 °C. Reaction check via HPTLC: reaction ended (only a halo is observed, which corresponds to the starting product). The reaction mixture is transferred to a solution of 375 mL glacial acetic acid in 750 mL water; exothermy (temperature from RT to 40 °C) and formation of a precipitate are observed during the addition. The pH is checked and found to be about 4. The solution is cooled to 0 °C for 1 hour, filtered and dried in stove at T = 60 °C for 16 hours, yielding 34.53 g of product. Mother liquor is re-extracted with 500 mL AcOiPr (1 time 300 mL, 2 times 100 mL). The solvent is distilled under reduced pressure at T = 50 °C. 150 mL water is added under stirring, observing the formation of a precipitate. The suspension is cooled to T = 0 °C stirring for at least 10 minutes. The solid is filtered and dried in stove at T = 60 °C for 16 hours, yielding 8.93 g of product. 260 mL isopropyl alcohol (6 volumes) is added to the crude product (two combined jets). The mixture is heated under reflux up to complete dissolution and it is then cooled to 0 °C for at least 1 hour. The solid is filtered and dried in stove at T = 60 °C, yielding 40.21 g of compound (III). Compound (II) analysis IR (KBr): 3473 cm-1; 1732 cm-1; 1647 cm-1; 1620 cm-1 Mass (El): M+ = 302; M+-H2O = 284 Molecular weight C19H26O3 = 302 Compound (III) analysis IR (KBr): 1.737 cm-1; 1.703 cm-1; 1.662 cm-1; 1.608 cm-1 Mass (CI): M++1 = 301 ; M++1-H20 = 283; M++1-2H20 = 265 Molecular weight C19H24O3 = 300
References:
WO2014/37873,2014,A1 Location in patent:Page/Page column 8; 9
19882-75-8
1 suppliers
inquiry

100071-90-7
9 suppliers
inquiry