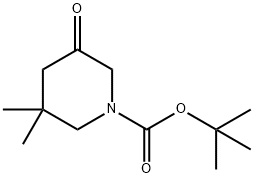
tert-butyl 3,3-dimethyl-5-oxopiperidine-1-carboxylate synthesis
- Product Name:tert-butyl 3,3-dimethyl-5-oxopiperidine-1-carboxylate
- CAS Number:1000894-83-6
- Molecular formula:C12H21NO3
- Molecular Weight:227.3
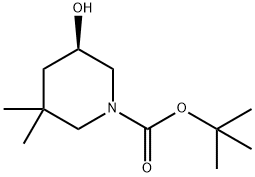
1189570-45-3
18 suppliers
$317.00/250mg

1000894-83-6
31 suppliers
$160.00/10mg
Yield:-
Reaction Conditions:
with Dess-Martin periodane in dichloromethane at 20;
Steps:
8.4
Step 4: To a RT solution of 126 (3.3 g, 15 mmol) in EtOH (100 mL) was added under N2 0.5 g of 20% Pd(OH)2/C (15% w/w). The reaction vessel was flushed with H2 and stirred 18 h. The reaction mixture was filtered through celite and concentrated in vacuo to an oil. To a RT solution of this aminoalcohol intermediate (1.39 g, 10.75 mmol) in THF (15 mL) was added NaOH (15 mL) followed by (Boc)2O (1.05 equiv, 11.3 mmol, 2.47 g). The reaction mixture was stirred at RT 18 h, then diluted with EtOAc and washed with water then brine. The organic layer was dried over MgSO4, filtered and concentrate in vacuo. The material crystallized upon standing to provide 1.9 g of the intermediate Boc-protected aminoalcohol. To a RT solution of this alcohol (8.3 mmol) in DCM (50 mL) was added Dess-Martin reagent (2 equiv, 16.6 mmol, 8 g). The reaction mixture was stirred overnight, then diluted with EtOAc and stirred with a 1/1 solution of Na2S2O3-NaHCO3 sat. The organic layer was separated, washed with NaHCO3 then brine, dried over MgSO4, filtered and concentrated in vacuo. Purification HPFC, 40+M, 5 to 20% EtOAc. After purification, 1.53 g of 127 was isolated (62% isolated yield over 3 steps).
References:
Schering Corporation US2008/4287, 2008, A1 Location in patent:Page/Page column 90