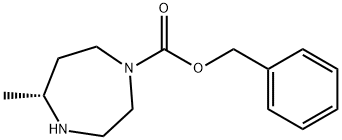
1H-1,4-Diazepine-1-carboxylic acid, hexahydro-5-Methyl-, phenylMethyl ester, (5R)- synthesis
- Product Name:1H-1,4-Diazepine-1-carboxylic acid, hexahydro-5-Methyl-, phenylMethyl ester, (5R)-
- CAS Number:1001401-60-0
- Molecular formula:C14H20N2O2
- Molecular Weight:248.32

217972-87-7
25 suppliers
$339.00/250mg
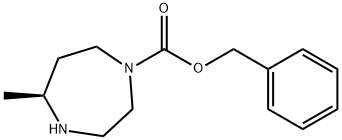
1001401-61-1
4 suppliers
inquiry

1001401-60-0
85 suppliers
$56.00/1g
Yield:> 95 % ee , > 95 % ee
Reaction Conditions:
in methanol;ethanol;hexane;Purification / work up;Resolution of racemate;
Steps:
SCHEME B
References:
WO2008/8518,2008,A1 Location in patent:Page/Page column 33

217972-87-7
25 suppliers
$339.00/250mg

1001401-60-0
85 suppliers
$56.00/1g
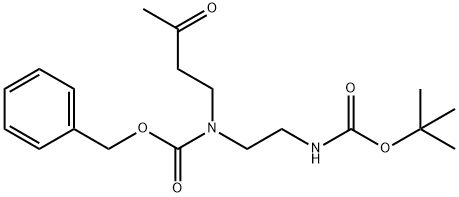
1030377-23-1
1 suppliers
inquiry

1001401-60-0
85 suppliers
$56.00/1g