
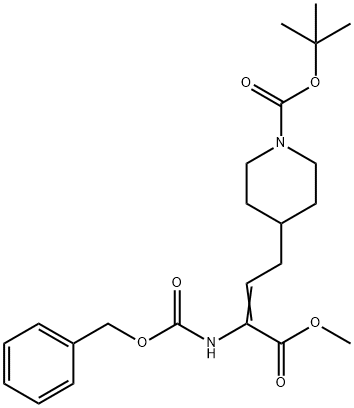
(R)-1-BOC-4-(3-CBZ-AMINO-3-METHOXYCARBONYL-PROPYL)PIPERIDINE synthesis
- Product Name:(R)-1-BOC-4-(3-CBZ-AMINO-3-METHOXYCARBONYL-PROPYL)PIPERIDINE
- CAS Number:1001646-85-0
- Molecular formula:C23H34N2O6
- Molecular Weight:434.53
![1-Piperidinecarboxylic acid, 4-[(2Z)-4-methoxy-4-oxo-3-[[(phenylmethoxy)carbonyl]amino]-2-buten-1-yl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/871675-56-8.gif)
871675-56-8
0 suppliers
inquiry

1001646-85-0
10 suppliers
inquiry
Yield:> 99 % ee
Reaction Conditions:
with hydrogen;(+)-1,2-bis-((2S,5S)-2,5-diethylphospholano)benzene(cyclooctadiene) rhodium(I) trifluoromethanesulfonate in methanol at 20; under 3102.97 Torr; for 24 h;
Steps:
d; 20.E
A Parr vessel is charged with 20-D (1.0 g, 2.31 mmol) and MeOH (100 mL) under nitrogen. The solution is subjected to three cycles of vacuum and nitrogen bubbling, and the catalyst (R,R)-Ethyl-DuPHOS-Rh(COD) triflate is added (30 mg, 0.04 mmol). The mixture is placed under 60 psi of hydrogen gas at room temperature for 24 h. The conversion to 20-E is complete after 24 h with >99% e.e., the solvent is removed in vacuo, and the crude product is purified by silica gel chromatography (hexanes/EtOAc).
References:
US2007/275906,2007,A1 Location in patent:Page/Page column 24-25
890849-78-2
22 suppliers
$360.00/1g

1001646-85-0
10 suppliers
inquiry