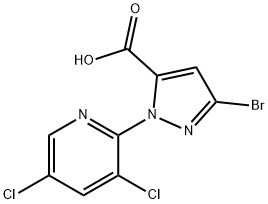
1H-Pyrazole-5-carboxylic acid, 3-broMo-1-(3,5-dichloro-2-pyridinyl)- synthesis
- Product Name:1H-Pyrazole-5-carboxylic acid, 3-broMo-1-(3,5-dichloro-2-pyridinyl)-
- CAS Number:1006620-93-4
- Molecular formula:C9H4BrCl2N3O2
- Molecular Weight:336.96
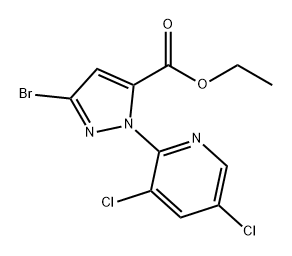
1104384-34-0
0 suppliers
inquiry

1006620-93-4
1 suppliers
inquiry
Yield:1006620-93-4 48%
Reaction Conditions:
Stage #1: ethyl 3-bromo-1-(3,5-dichloropyridin-2-yl)-1H-pyrazole-5-carboxylatewith water;sodium hydroxide in methanol at 20; for 1 h;
Stage #2: with hydrogenchloride in water; pH=4;
Steps:
4.3
(3) Synthesis of 3-bromo-1-(3,5-dichloropyridin-2-yl)-1H-pyrazole-5-carboxylic acid To a 100 mL flask, ethyl 3-bromo-1-(3,5-dichloropyridin-2-yl)-1H-pyrazole-5-carboxylate (2.0 g, 5.5 mmol), methanol (10 mL), water (10 mL) and sodium hydroxide (0.3 g, 5.5 mmol) were added. After being stirred at room temperature for 1 hour, the mixture reacted completely. The reaction mixture was concentrated by rotary evaporator to about 10 mL as a dark brown solution. Then water (40 mL) was added into the dark brown solution. The aqueous solution was extracted with ethyl ether (50 mL) and acidified with concentrated hydrochloric acid to pH of 4. The precipitate was isolated by filtration, washed with water (2×50 mL) and dried to give the product (0.97 g) as white solid in 48% yield.1H NMR (300 MHz, DMSO-d6): 8.641 (d, 1H), 8.529 (d, 1H), 7.186 (s, 1H).
References:
US2011/46186,2011,A1 Location in patent:Page/Page column 13

1104384-33-9
0 suppliers
inquiry

1006620-93-4
1 suppliers
inquiry

1104384-27-1
0 suppliers
inquiry

1006620-93-4
1 suppliers
inquiry
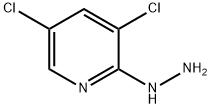
104408-23-3
33 suppliers
inquiry

1006620-93-4
1 suppliers
inquiry
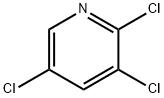
16063-70-0
376 suppliers
$6.00/5g

1006620-93-4
1 suppliers
inquiry