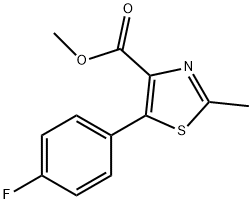
METHYL 5-(4-FLUOROPHENYL)-2-METHYLTHIAZOLE-4-CARBOXYLATE synthesis
- Product Name:METHYL 5-(4-FLUOROPHENYL)-2-METHYLTHIAZOLE-4-CARBOXYLATE
- CAS Number:1007873-79-1
- Molecular formula:C12H10FNO2S
- Molecular Weight:251.28
Yield:1007873-79-1 58%
Reaction Conditions:
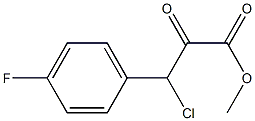
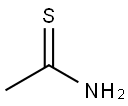
Stage #1: methyl 3-chloro-3-(4-fluorophenyl)-2-oxopropanoate;thioacetamide in methanol; for 1.16667 h;Reflux;
Stage #2: with sodium hydrogencarbonate in dichloromethane;water;ethyl acetate at 20;
Steps:
3.b
To a refluxing solution of thioacetamide (9.02 g) in dry MeOH (200 mL) was added dropwise over 10 min. a solution of 3-chloro-3-(4-fluoro-phenyl)-2-oxo-propionic acid methylester (31.30 g) in dry MeOH (50 mL). The reaction mixture was stirred at reflux for 1 h. After cooling to RT, the reaction mixture was concentrated in vacuo and the residue was diluted with DCM (500 mL) and EA (150 mL). The solution was washed with cold water (100 mL), sat. NaHCO3 solution (100 mL). The organic phase was washed again with sat. NaHCO3 solution (100 mL), water (2×50 mL), dried (Na2SO4), filtered and concentrated to yield a crude oil.FC (EA/petroleum ether: 30/75 to 1/2) gave 17.71 g (58%) of the title compound.1H-NMR (CDCl3): δ=2.75 (s, 3H); 3.84 (s, 3H); 7.10 (m, 2H); 7.47 (m, 2H).
References:
US2010/222600,2010,A1 Location in patent:Page/Page column 23

459-57-4
582 suppliers
$6.00/25g

1007873-79-1
4 suppliers
inquiry