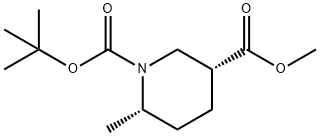
1-tert-butyl 3-methyl (3R,6S)-6-methylpiperidine-1,3-dicarboxylate synthesis
- Product Name:1-tert-butyl 3-methyl (3R,6S)-6-methylpiperidine-1,3-dicarboxylate
- CAS Number:1009377-05-2
- Molecular formula:C13H23NO4
- Molecular Weight:257.33

24424-99-5
839 suppliers
$13.50/25G

1009376-81-1
29 suppliers
inquiry

1009377-05-2
8 suppliers
inquiry
Yield:1009377-05-2 71%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20; for 19 h;
Steps:
Preparation of (3R,6S)-1-tert-Butyl 3-Methyl 6-Methylpiperidine-1,3-dicarboxylate (6-303β2-Me) To a solution of 6-313β2-Me (14 g) in CH2Cl2 (287 mL) and Et3 N (50 mL, 4 equiv) was added 23.3 g (1.2 equiv) of Boc2O at 0° C. After stirring at room temperature for 19 hours, GC-MS analysis indicated <1% of 6-313β1-Me remained. The reaction mixture was diluted with DI water (90 mL) and the phases were separated. The CH2Cl2 phase was washed with DI water (90 mL), dried (Na2SO4), filtered and concentrated to a residue. The residue was azeotroped with hexanes (2*250 mL) and purified by silica-gel chromatography (99:1 n-heptane/ethyl acetate) to afford 6-303β2-Me (16.3 g, 71%).
References:
US2010/93706,2010,A1 Location in patent:Page/Page column 51-52